Internal mammary artery perforator (IMAP) flap plus superior epigastric artery perforator (SEAP) flap as salvage breast reconstruction after multiple surgical failures: a case report
Highlight box
Key findings
• Autologous salvage breast reconstruction with “uncommon” perforator flaps is a valid alternative to implant-based and microsurgical breast reconstruction.
What is known and what is new?
• Salvage breast reconstruction aims to reproduce the breast mound after previous, failed reconstructive attempts and can be achieved through the use of implants or autologous tissue transfer.
• Usually, local perforator flaps are not the first choice when it comes to breast reconstruction, in favor of microsurgical flaps. Nevertheless, they are an option in case of salvage breast reconstruction.
What is the implication, and what should change now?
• Salvage breast reconstructions are always challenging for surgeons. A thorough pre-operative study and listening to patient’s desires are mandatory for successful results.
Introduction
Post-mastectomy breast reconstruction is a procedure that aims to recreate an aesthetically pleasant and proportionally sized breast mound. It can be primary, when the reconstruction is performed immediately after mastectomy; secondary (delayed) when mastectomy and reconstruction are carried out during two different surgeries; or tertiary (salvage), when the reconstruction follows a previous, unsatisfactory, or failed reconstructive attempt (1). The latter is different from small touch-up surgeries that are usually carried out to improve the final aesthetic result. Complication rates following implant-based breast reconstruction can be as high as 35–60%, with additional surgery and explantation necessary in 3% of patients. Salvage breast reconstruction can become a reconstructive challenge, providing emotional distress for both the surgeon and the patient, who may be anxious about the outcomes after many surgeries. Furthermore, every surgery adds scars and compromises the vasculature, making it difficult to re-operate on the same body area. The salvage restoration of the breast mound can be achieved with an implant, autologous tissue, or a combination of both (2,3).
With this case, we aim to demonstrate that complex cases sometimes require complex solutions which can be managed applying “common” solutions in peculiar ways. We present this case in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-23-37/rc).
Case presentation
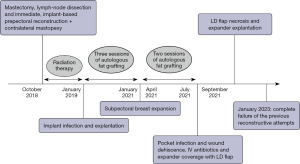
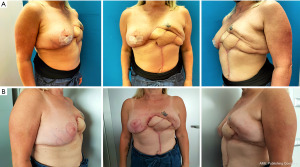
We describe here the case of a 52-year-old woman who came to our attention after several unsuccessful attempts at post-mastectomy breast reconstruction. The patient is a non-smoker, healthy woman with a body mass index (BMI) of 22.4 kg/m2 and a history of infiltrating ductal carcinoma of the left breast. No recurrence nor metastases were detected on pre-operative ultrasonography and mammography bilaterally. In October 2018, she underwent left mastectomy and axillary lymph node dissection and immediate, implant based, acellular dermal matrix (ADM)-assisted prepectoral breast reconstruction and contralateral mastopexy. The patient was then treated with adjuvant radiotherapy because of lymph-node involvement. In January 2019, the implant was removed because of infection and wound dehiscence and, after full recovery, the mammary region was treated with three sessions of autologous fat grafting. In January 2021, a subpectoral breast expander was positioned and other two sessions of autologous fat grafting were carried out between April and July 2021, when she experienced infection of the breast implant again. After administration of parenteral antibiotics, the subpectoral pocket was revised and the implant was covered using a latissimus dorsi (LD) flap. Unfortunately, she experienced necrosis of the medial tip of the LD flap, which was discarded together with the breast expander in September 2021 (Figure 1). The patient presented at our department asking for a “salvage” breast reconstruction. Given that she had already undergone four previous surgeries, she refused to undergo complex or time-consuming surgeries like free-flap reconstruction, so we had to plan an alternative based solely on loco-regional flaps that could still provide acceptable results. This was particularly troublesome since the patient presented numerous scars on the operated mammary region with an oblique, retractive scar crossing the mammary region from the sternum to the left anterior axillary line. The lateral portion of the breast retained some of the volume previously provided by the LD flap and the skin here presented the typical consistency and structure of the dorsal cutaneous coverage. The medial and inferior portions of the breast had been replaced by a central retractive scar surrounded by stiff, scar-like tissue with no volume nor projection. The nipple-areola-complex had been excised during the mastectomy. No palpable masses were detected (Figure 2).
The patient’s anterior chest wall was explored using a hand-held color Doppler ultrasound probe in search of viable perforators. The internal mammary artery perforators (IMAPs) on the contralateral side were undamaged and of adequate caliber, and so was the superior epigastric artery perforator (SEAP) on the operated side. Thanks to the availability of safe perforators and respecting the patient’s wishes, we chose to perform the reconstruction using a combination of perforator flaps. Despite the previous reduction mammoplasty, the contralateral breast could still provide a small amount of gland and subcutaneous fat to compensate for the discrepancy of volume between the two sides. We then decided to transpose the excess tissue on the healthy side to the contralateral side in a pedicled fashion to add volume to the affected side and reduce the breast mound on the contralateral. The first surgery was carried out in January 2023. After identification of an IMAP of adequate caliber, a gland-cutaneous flap was raised and transposed to the contralateral chest wall, adding volume to the upper-medial portion of the breast mound. The inferior aspect of the breast was reconstructed using a classical SEAP flap, which was inset after thorough revision of the scarred tissue on the anterior chest wall. The remaining portion of the LD flap was left in place to provide volume to the lateral aspect of the breast.
Four months later (May 2023), the patient was readmitted to our department for the first touch-up surgery. The IMAP and SEAP flaps were revised in order to recreate the inframammary fold and correct the sinmastia. Laterally, the residual LD flap was de-epithelialized, buried under the SEAP flap and moved medially to add volume to the breast mound. Additionally, 200 cc of fat (harvested from the flank regions) was grafted to the IMAP and SEAP flaps to add further volume and to give a rounder shape to the breast.
One-month post-operative the breast mound profile appeared dramatically improved, with little to no fat resorption (Figure 3).
Undoubtedly, other sessions of fat grafting and flap remodeling will be necessary to achieve a satisfactory result, but this combination of local flaps has given us the possibility to create a safe scaffold for further refinements.
At the 3-month post-operative follow-up evaluation the patient is aware that other surgeries will probably be necessary to achieve an aesthetically pleasant result, but at the moment she is satisfied with the results achieved so far.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Implant-based reconstruction is a cornerstone in breast reconstruction and, in some countries, it is the most frequent modality of breast reconstruction (4,5). The advantages of this type of reconstruction include simple device placement, reduction of operative times and lack of donor-site morbidity. Unfortunately, implants act as foreign bodies and, as so, they are burdened with risks and possible complications which include infection, seroma formation, capsular contracture, rippling, and implant rupture.
Additionally, in the last decades radiotherapy has gained a central role in breast cancer treatment, increasing the number of patients receiving post-surgical chest-wall irradiation. Consequently, many recent studies have addressed the potentially negative effects of radiotherapy on breast implants, generally discouraging their use when post-mastectomy adjuvant radiation therapy is foreseen. The most common complications after implant-based breast reconstruction are: capsular contracture, infection, wound dehiscence and implant extrusion, and complication rates seem to be higher after radiotherapy (6-10). Although implant-based tertiary breast reconstruction is still a feasible option in selected cases (11), most studies agree in considering autologous breast reconstruction as the best salvage option. The use of autologous flaps following failed implant reconstruction usually results in improved cosmesis, natural appearance of the breast and resolution of pre-existing symptoms, with low morbidity and complication rates. Given the presence of scar tissue, compromised vasculature or previous radiotherapy, salvage autologous breast reconstruction is slightly more risky than primary and secondary flap reconstructions, so that accurate preoperative planning including appropriate imaging and screening for pre-existing diseases is mandatory.
This case is an example of “alternative” salvage breast reconstruction in which two different perforator flaps were combined to give the patient an aesthetically pleasant result.
The SEAP flap, first described by Hallock in 2005 (12) and then made famous by Hamdi (13) in 2009 is the perforator derivative of the transverse rectus abdominis myocutaneous (TRAM) flap and is quite commonly used to reconstruct defects of the anterior thoracic wall. Differently from its myocutaneous counterpart, the SEAP flap spares the whole transverse rectus abdominis muscle and fascia, reducing donor-site morbidity yet providing adequate coverage of anterior chest wall defects. Nevertheless, the SEAP flap has rarely been taken into account for breast reconstruction, in favor of another perforator flap supplied by the epigastric artery system, the deep inferior epigastric perforator (DIEP). The DIEP flap is nowadays considered the gold standard in autologous breast reconstruction, thanks to the amount of tissue it provides, the possibility of a like-with-like reconstruction and the persistency of the result over time. On the other side, the SEAP has been used in partial breast reconstruction mainly as a pedicled flap, particularly when reshaping the breast’s lower aspects. In the literature, there are few cases of complete breast reconstruction in which a free SEAP flap was used, but they are all cases in which the deep inferior epigastric artery (DIEA) was injured or a previous DIEP flap was raised (14,15). The IMAP flap is a flap in which vascular supply arises from the perforating branches of the internal mammary artery. Thanks to its great variability, it has been used mainly for the reconstruction of the anterior chest wall but also head and neck reconstruction (16,17). In 1973, Pontes et al. first described a “breast sharing technique” in which the otherwise discarded dermo-glandular tissue deriving from a breast reduction was transposed to a volume-deficient contralateral side (18). Other authors (19,20) have later described their own techniques for breast sharing. However, it was only after the description of the perforators system that the anatomical basis for this type of procedure was clarified. The “breast sharing technique” is nonetheless an IMAP flap based on the perforator of the fourth intercostal space which usually supplies a large angiosome of skin between the areola and the inframammary fold.
To our knowledge, this is the first case in which a combination of IMAP flap + SEAP flap was used for complete restoration of the breast mound. The decision to employ two local pedicled perforator flaps was made partly because the patient rejected the opportunity of a free-flap reconstruction; but mostly because we think that microsurgery should be considered the gold standard only when considering primary or secondary breast reconstruction. In cases like the one here described, when great tissue remodeling and scarring are present because of previous breast surgeries, a microsurgical approach could further complicate an already treacherous situation, adding unnecessary emotional burden for both the surgeon and the patient.
Conclusions
Perforator flaps have provided great freedom to plastic surgeons, who can apply them as desired depending on each particular reconstructive demand. After a thorough evaluation of the patient’s needs and expectations, it is sometimes possible to employ perforator flaps in “exotic” ways, overcoming challenging situations yet avoiding more complex reconstructive solutions. We decided to share this case for the peculiarity of its presentation and the complexity of its management. The patient had really clear ideas about what she wanted and what she would not accept as a reconstructive option. This limited greatly our reconstructive options. Additionally, she had already undergone four different surgeries which had widely subverted the tissues’ anatomy and consistency.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-23-37/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-37/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-37/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hamdi M, Casaer B, Andrades P, et al. Salvage (tertiary) breast reconstruction after implant failure. J Plast Reconstr Aesthet Surg 2011;64:353-9. [Crossref] [PubMed]
- Mohan AT, Al-Ajam Y, Mosahebi A. Trends in tertiary breast reconstruction: literature review and single centre experience. Breast 2013;22:173-8. [Crossref] [PubMed]
- Hassan AM, Tran J, Asaad M, et al. Outcomes of Third-Attempt Breast Reconstruction following Infection-Associated Failure of Secondary Implant-Based Reconstruction. Plast Reconstr Surg 2023;151:367e-75e. [Crossref] [PubMed]
- The National Mastectomy and Breast Reconstruction Audit. A National Audit of Provision and Outcomes of Mastectomy and Breast Reconstruction Surgery for Women in England. Fourth Annual Report. 2011. Available online: https://digital.nhs.uk/catalogue/PUB02731
- American Society of Plastic Surgeons. 2020 National plastic surgery statistics. Available online: https://www.plasticsurgery.org/documents/News/statistics/2020/plastic-surgery-statistics-report-2020.pdf
- Emanuele Lisa AV, Salgarello M, Huscher A, et al. The Effect of Adjuvant Radiotherapy on One- and Two-Stage Prosthetic Breast Reconstruction and on Autologous Reconstruction: A Multicenter Italian Study among 18 Senonetwork Breast Centres. Breast J 2023;2023:6688466. [Crossref] [PubMed]
- Coriddi M, Shenaq D, Kenworthy E, et al. Autologous Breast Reconstruction after Failed Implant-Based Reconstruction: Evaluation of Surgical and Patient-Reported Outcomes and Quality of Life. Plast Reconstr Surg 2019;143:373-9. [Crossref] [PubMed]
- Levine SM, Lester ME, Fontenot B, et al. Perforator flap breast reconstruction after unsatisfactory implant reconstruction. Ann Plast Surg 2011;66:513-7. [Crossref] [PubMed]
- Kroll SS, Freeman P. Striving for excellence in breast reconstruction: the salvage of poor results. Ann Plast Surg 1989;22:58-64. [Crossref] [PubMed]
- Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg 2000;105:930-42. [Crossref] [PubMed]
- Visser NJ, Damen THC, Timman R, et al. Surgical results, aesthetic outcome, and patient satisfaction after microsurgical autologous breast reconstruction following failed implant reconstruction. Plast Reconstr Surg 2010;126:26-36. [Crossref] [PubMed]
- Hallock GG. The superior epigastric(RECTUS ABDOMINIS) muscle perforator flap. Ann Plast Surg 2005;55:430-2. [Crossref] [PubMed]
- Hamdi M, Van Landuyt K, Ulens S, et al. Clinical applications of the superior epigastric artery perforator (SEAP) flap: anatomical studies and preoperative perforator mapping with multidetector CT. J Plast Reconstr Aesthet Surg 2009;62:1127-34. [Crossref] [PubMed]
- Kundu N, Chopra K, Morales R, et al. Superior epigastric artery perforator (SEAP) flap: a novel approach to autologous breast reconstruction. J Plast Reconstr Aesthet Surg 2015;68:519-24. [Crossref] [PubMed]
- Mountziaris PM, Patel A, Rezak KM. Breast reconstruction with superior epigastric artery perforator (SEAP) free flap: Report of two cases. Microsurgery 2020;40:593-7. [Crossref] [PubMed]
- Faini G, Pierazzi DM, Arleo S, et al. Internal mammary artery perforator flap for anterior thoracic and upper abdominal wall reconstruction: 16 case series J Plast Reconstr Aesthet Surg 2022;75:2387-440. [Crossref] [PubMed]
- Bachleitner K, Weitgasser L, Amr A, et al. Autologous Unilateral Breast Reconstruction with Venous Supercharged IMAP-Flaps: A Step by Step Guide of the Split Breast Technique. J Clin Med 2020;9:3030. [Crossref] [PubMed]
- Pontes R. Single stage reconstruction of the missing breast. Br J Plast Surg 1973;26:377-80. [Crossref] [PubMed]
- Marshall DR, Anstee EJ, Stapleton MJ. Post mastectomy breast reconstruction using a breast sharing technique. Br J Plast Surg 1981;34:426-30. [Crossref] [PubMed]
- Morritt AN, Grinsell D, Morrison WA. Postmastectomy breast reconstruction using a microvascular breast-sharing technique. Plast Reconstr Surg 2006;118:1313-6. [Crossref] [PubMed]
Cite this article as: Faini G, Bonetti C, Valdatta L, Arleo S. Internal mammary artery perforator (IMAP) flap plus superior epigastric artery perforator (SEAP) flap as salvage breast reconstruction after multiple surgical failures: a case report. AME Surg J 2023;3:50.