
A perspective on lung metastasectomy: a review of a flawed concept and the failure to use available evidence resulting in an illusion of benefit
Introduction
Beliefs in the effectiveness of local treatment of pulmonary metastases and current practice are based on the claims for a large survival difference. Rather than accepting the status quo, we question and challenge it. Our objective is to point to the weakness of the evidence for metastasectomy and suggest that the consensus of belief of American and European thoracic surgeons is evidence of optimism bias and competing interests at work (1,2).
We present our arguments in three parts:
- Taking the long view, we give examples of beliefs and practices from the time of our great grandparents to remind readers of how fundamentally belief and practice change over time.
- Then, we consider how our present day practice might look from the perspective of researchers in the future.
- Finally, we offer proposals for how to work towards more rational and evidence based practice.
Part one: taking the long view
The nature of surgery—its safety and effectiveness—has changed greatly in our lifetimes. Clinical practice responds to new knowledge but it is important to recognise that it is not relentless “progress”. There are retreats from accepted practice, but those treating lung metastases with surgery or ablation believe that are really doing the best for their patients. That is exactly what doctors at the time of their great grandfathers also believed. But their treatments were very different. They did not have the scientific knowledge and technology that we have, but they were as intelligent, compassionate and professionally committed as today’s doctors, and yet much of what they did we would not dream of doing now. And so, three generations into the future what will be the accepted treatment for a patient with a few lung metastases? It is probable that it will have changed. Change might be due to cancer treatment that we cannot yet imagine or it may be that future doctors will see today’s piecemeal eradication of metastases as futile and wrong-headed.
In the course of an historical project (3) a focussed search was made of the contents of the British Medical Journal (BMJ) and The Lancet from the 1890s to the 1920s. These journals were published weekly and they were widely read. There are many examples of treatments replaced by something better, many stopped because they were found to be ineffective, and others were recognised to do more harm than good. Four instances, recognisable by thoracic surgeons, are chosen to illustrate how and why change happened.
Pulmonary tuberculosis
The change
A century ago tuberculosis was managed in public health funded sanatoria. It was in the operating theatres of these institutions that interventions such as artificial pneumothorax, plombage, thoracoplasty and selective lung resection were practised and refined as the specialty of thoracic surgery emerged and grew. The discovery of streptomycin and its introduction after a controlled trial rendered most of surgery for tuberculosis obsolete (4).
Historical context
Tuberculosis (TB) was endemic throughout Europe and America at the time of our great grandfathers. The British Medical Association (BMA) held annual week-long summer meetings and in 1899 the leading topic was TB (5). The mainstays of treatment were fresh air, good feeding and rest in health resorts or designated sanatoria. All appeared to have some success but which was best? A letter in the BMJ in the same year, 1899, sums it up: “Neither Switzerland, the Riviera, Egypt, the sea, or an English veranda, can justly claim patent right for the treatment of phthisis [pulmonary tuberculosis]. Any of them may be statistically shown to be the best if the cases they treat are selected with sufficient care, and especially if their failures are quietly sent elsewhere.” (6).
The author was referring to the misleading nature of claims of benefit without denominators, of case selection and no controlled data.
Management of the pleura
The change
Understanding and managing the pleura is taken for granted in the practice of thoracic surgery but breaching the pleura was life-threatening three generations ago. Tracheal intubation, positive pressure ventilation and water seal drainage, came about due to technical advances, each of which, once mastered and its utility repeatedly confirmed, needed no further proof of effectiveness. These are interventions for which the parachute analogy can reasonably be invoked: the mechanism is obvious and the lifesaving effect is immediately self-evident (7).
Historical context
Breathlessness due to “water on the lung” was well recognised. In the BMJ and The Lancet 1904–1907 there were lengthy exchanges of correspondence with claims and counterclaims about how to manage pleural effusion (8,9). The editors halted the correspondence because the arguments became heated and personal (10). From the perspective of our present day knowledge of the pleura and its pathology, it is apparent that they were dealing with a number of different aetiologies. Pleural effusions might have been infected from the outset or at high risk of becoming so, but in their time, they had no imaging, and they were handicapped by not having a shared vocabulary with consistent meanings. Amongst the letters is one from a surgeon in 1907 about draining empyema which stands out for its clarity of purpose and technique (11).
In the 1914–1918 war an isolated chest wound was the cause of death in a third of all killed on the battlefield. Of those reaching medical care, sixty percent still died mainly due to failure to manage the pleural space. The change in understanding and managing the pleural space, unlike the effectiveness of streptomycin, was not be due to controlled trials. It was due to the application of physics and physiology, to devise methods repeatedly seen to be rapidly beneficial in clinical practice.
Mitral stenosis
The change
Operating on the heart was explicitly ruled out by mainstream medical opinion until after 1948 when mitral valvotomy was shown to immediately relieve breathlessness and improve exercise tolerance. It became the standard operation, undertaken throughout the world (12). As heart surgery developed in the hands of thoracic surgeon over the next 15–20 years, dedicated cardiac surgical practice emerged.
Historical context
Reporting the finding of 196 patients who died with mitral stenosis in Guy’s Hospital an article in The Lancet in 1898 concluded: “I anticipate that with the progress of cardiac surgery some of the severest cases of mitral stenosis will be relieved.” (13,14). The idea resurfaced several times in the following 50 years but it was met with ever increasing derision by physicians. They firmly believed that operating on the heart would be madness. They regarded the narrowed valve as a mere epiphenomenon and the heart muscle as the real problem (12,15). Following the D-Day landings in 1945, Dwight Harken working in a US army hospital in rural England, performed 139 operations removing ironmongery from within and around the heart without a single death. In 1948 Harken, soon followed by other thoracic surgeons, proved the 1898 prediction that surgeons would relieve mitral stenosis. In the decade before pump oxygenators became available, thoracic surgeons performed mitral valvotomy on the beating heart, to the benefit of thousands of patients.
Surgical relief of mitral stenosis was very definitely a condition for which the parachute analogy can be invoked (16). The problem and its relief are mechanically explicable and the benefit was great. This is in marked contrast to asymptomatic lung metastasectomy from which the patients never derive direct symptomatic benefit and survival time is confounded by other factors.
Bloodletting
Treatment by venesection was a panacea which is now generally regarded as an example of craziness in the history of medicine. It slipped quietly “out of fashion” without having to be disproved (17).
In summary, the glimpses of history given in this section include two diseases which were endemic—tuberculosis and mitral stenosis. Thoracic surgeons became central to their treatment. For treatment, we have a much clearer understanding of pleural disease and pleural complications resulting from treatments but the pleura can still pose very difficult problems. The fourth example, bloodletting, was going out of fashion over 100 years ago. All four of those areas of medical practice look very different now.
Part two: the present evidence for lung metastasectomy as it might be viewed by researchers in the future
We can only imagine what the practice of medicine will be in the time of our great grandchildren. Some of them are likely to be curious about how our practice compares with theirs. We are hopeful that in the intervening years discoveries will radically change the understanding of cancer and its treatment. We cannot know but anticipate that piecemeal removal of metastatic cancer by surgical removal or ablations will have passed into history.
In part one, we “took the long view” to show—with glimpses into the history of tuberculosis, surgical management of the pleura, heart disease and bloodletting—how conventional medical practice has changed fundamentally. The changes happened for different and largely unpredictable reasons. Looking backwards we can see how new knowledge and technical achievements transformed practice. Future researchers will be able to search the literature of a century ago. Let us look at this question from their perspective by giving present day readers a guided tour of what they will be able to find.
A search of the literature for metastasectomy up to the present returns nearly a thousand titles. Researchers will no doubt narrow their searches to find papers which suit their individual research questions according to Types A, B and C:
- A: surgeons in clinical practice;
- B: epidemiologists, health service researchers, economists and medical statisticians;
- C: professional historians.
Type A researchers: surgeons in clinical practice
In the year 2123, clinical researchers with an interest in the evidence for practice might search specifically for randomised controlled trials (RCTs). They will find just one—Pulmonary Metastasectomy for Colorectal Cancer (PulMiCC)—described in a cluster of papers (18-23). They will see that it was funded by a charity called Cancer Research UK. The joint grant holders were an academic psychologist and a surgeon, with the close involvement of oncologists, statisticians and trials scientists. The study was run by the Royal Brompton Hospital and University College London. There were 25 international clinical sites involved, one each in China, Italy and Serbia and the rest in England. Compared with the nature of contemporary research this study might seem to researchers of the future to be at the more credible end of the range of published literature returned by their searches.
The total enrolment was 512 patients who fell into three categories.
- There were 28 excluded because their lung nodules proved to be benign or a non-colorectal pathology (18);
- An offer of randomisation was made and accepted by 93 patients (19);
- For 391 a clinical decision was made, 263 for and 128 against lung surgery.
The 5-year survival of these elective groups is shown in the upper panel of Figure 1. For those selected for metastasectomy survival was similar to the highest survival rates among the follow-up studies included in a meta-analysis (24). Future researchers will recognise this as giving “face validity” to the PulMiCC study.

Case report forms (CRFs) collected at the outset of the study provided information on oncological prognostic factors—number of metastases, carcinoembryonic antigen (CEA) assay, interval since primary resection, stage of the resected primary, whether there was liver involvement—and the patients’ age, sex, height, weight and lung function. The process of randomisation included minimisation ensuring that the two arms of the randomised trial were balanced (19). In the RCT operated and control patients were similar (lower panel). There was no evident survival benefit from metastasectomy. The median survival was actually longer in the control arm (3.8 versus 3.5 years).
Type B: epidemiologists, health service researchers, economists and medical statisticians
The statistically minded scientists of the future might be interested in the growth of “Big Data” research in our time. Of particular interest might be an analysis of the Surveillance, Epidemiology and End Results (SEER) database (25). Data on 10,325 patients were “analyzed by Cox regression with multivariable, inverse propensity weight, near far matching and propensity score adjustment” (25). And what was the answer? For patients with lung metastases, whether they had metastasectomy or not there was no statistical difference in survival (hazard ratio =0.84, 95% CI: 0.62–1.12, P=0.232).
Numerically literate researchers of the future might wonder how the illusion, that lung metastasectomy saves lives, was sustained when both an RCT (19) and a “Big Data” analysis (25), both published in 2020, precluded any substantial effect. They will be able to gauge the strength of conviction on both sides of the Atlantic. From Europe in 2017 came the declaration that “Surgery for pulmonary metastases is a pillar of modern thoracic surgery” (26). From America in 2019 the assertion by consensus of thoracic surgeons that without metastasectomy “survival is assumed to be zero”. This implausibly low benchmark created the illusion that all patients alive at 5 years had surgeons to thank for their lives.
Selection of patients naturally destined to survive creates an illusion of effectiveness of the chosen treatment as implied by Dr. Samways in 1899 (6). The degree of selection can be approximated from published data available to researchers of the future. Of 22,715 patients who had colectomy at US Veterans Affairs hospitals between 1965 and 1988, 2,609 had lung metastases but only 76 patients (3%) had them resected (27). This was published in the 1990s when colorectal cancer (CRC) lung metastasectomy was taking off (28). Future searches will find a similar analysis 25 years later. In the British National Health Service 173,354 individuals had a major colorectal resection from 2005 to 2013 and of them 3,434 (2.0%) underwent a pulmonary resection within 3 years (29).
For lung metastasectomy for CRC two single institution follow-up studies of 144 and 159 lung reported 5 and 10-year survival. Like many other follow-up studies in this era, they provide no information about the possible denominator from which they were drawn (30,31). The operations were done during time windows of 24 and 28 years which averages out at one operation every two months. Thoracic surgical services were much more centralised than surgery for CRC so this reflects a very high degree of selection associated with 5-year survival rates of 40–50%. For example, more than 60% of the operations in these series were for a solitary metastasis.
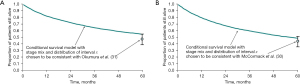
These two studies also provided the stage of the primary cancer and the interval since that operation, factors also recorded in the Thames Cancer Registry. The data were used in a modelling study using known survival of patients containing 39,112 patient records. The survival rates of matched groups of patients in the registry who had not had metastasectomy were similar to or better than those who had a metastasectomy in the follow-up studies (32,33) (Figure 2).
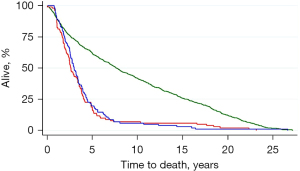
It is likely that screening protocols will be further studied and refined and they may well remain contentious. In the early days of using CEA to detect CRC recurrence the CEA Second Look (CEASL) trial was run to see if earlier detection of metastases would provide better survival. CEA elevation detected recurrence 11 months sooner than clinical observational but second look surgery provided no benefit (34). Disappointed, the surgical trialists shelved the data (Figure 3). It is probable that future researchers will discover this because the recovered data were re-analysed and published with a BMJ editorial 25 years later (35-37). Over the subsequent years 16 RCTs were run testing each iteration of more intensive secondary surveillance as new modalities were introduced. A survival benefit was never found as was confirmed in two meta-analyses, one of them a Cochrane study (38,39). Metastasectomy continued nevertheless.
Future researchers with a mathematical mindset will be able to find these four forms of evidence—mathematical modelling, meta-analysis of surveillance for early detection, a pragmatic RCT and a propensity matching study—that metastasectomy is not associated with higher survival than that of comparable patients who are simply observed (19,25,32,33,38,39). They will be able to find out when the practice dwindled, as we expect it will with new scientific knowledge. They may think that there was already sufficient evidence to bring it to a halt by 2023. We do.
Type C: professional historians
Historians are the least likely to be surprised by the rise and fall of local treatments, which they will regard as predictable, given the trajectory of so many treatments in the history of medicine. For them medical practice is a part of cultural history (40). The emergence of a new diagnostic frame of “oligometastasis” in 1995 might attract their attention (41).
Diagnoses depend on the methods available. In ancient times, diseases were recognised by the visible external features and so there were diagnoses such was apoplexy, dropsy and consumption. Consumption included “wasting” diseases where fat and muscle are “consumed” in the course of the disease. A wasting disease associated with coughing and spitting blood was called pulmonary consumption, or phthisis. In the era when “morbid anatomy” further categorised disease, the tubercles in the lung became a postmortem diagnostic feature. Koch’s discovery of the organism within the tubercles as the transmissible cause of the disease led him to name the germ Mycobacterium tuberculosis (42,43). Now the diagnosis is framed by the causative organism which distinguishes tuberculosis from (for example) sarcoid and squamous cell carcinoma which are also forms of cavitating lung disease associated with cough and weight loss.
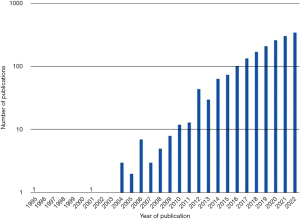
Oligometastasis is framed by the fewness of metastases and is reliant on modern imaging. The idea received almost no published attention for a decade (Figure 4) but was taken up by radiation oncologists and interventional radiologists, the latter to justify the use of image guided thermal ablation (IGTA). But oligometastasis is simply the tapering end of a frequency distribution which provides a therapeutic opportunity (44). As with surgical metastasectomy there were follow-up studies with no control groups or estimates of survival in similar patients who did not have local treatments.
Future historians might pick up on the consensus that the 5-year survival of around 40% was all attributable to their operations. Doctors assumed it would be zero without their intervention. A dozen authorities of the Society of Thoracic Surgeons (STS) put their name to this palpable falsehood, admitting that it was without evidence (1). They cited PulMiCC as “in progress” but disregarded its findings even though the report of the RCT was with the STS journal at the time, only to be rejected, thus delaying the publication of evidence while the unfounded conviction of its members appeared in print.
The publications from PulMiCC that might interest them most relate to the response of surgeons to a controlled trial which undermined their convictions. Historians like controversy between named prominent individuals. They might find instructive the head-to-head debate in the European Journal of Cardiothoracic Surgery between two prominent advocates of CRC lung metastasectomy and two protagonists of PulMiCC (45). The advocates of metastasectomy described a 10-year survivor who had three operations and repeated chemotherapy. Clearly the first two operations had failed in their objective of eradicating her cancer and the attribution of her survival to lung metastasectomy was not sustainable. The arrangement was to provide two case instances on each side but the “true believers” failed to present a second example.
Researchers of Types A, B and C might in their different ways be interested in a systematic review of the responses to PulMiCC. By October 2021, 64 publications had cited the RCT. They came from authors treating lung metastases with the large majority discounting the trial in a line or two (23). But in the words of Paul Simon “a man hears what he wants to hear and disregards the rest”. Least surprised will be the social historians who are aware of the human factors such as cognitive bias and competing interests, which have allowed the rise and fall of strongly held but misguided beliefs.
Part three
Special cases
For many people in present times, cancer is their greatest fear and—once the belief that detection and piecemeal eradication might postpone death is implanted in their psyche—many will go to any lengths to have it detected and eradicated from their bodies. Here we specifically consider special cases—sarcoma, germ cell, and trophoblastic tumour—for which operation as part of multimodality treatment is likely to persist.
Lung metastasectomy for sarcoma
The foundations of lung metastasectomy were formally set out more than 50 years ago by surgeons at Memorial Sloan Kettering (MSK) who began to offer operations for metastases from sarcoma (46). The justification given was that “More than 80% of patients with osteogenic sarcoma develop pulmonary metastases within two years and, if untreated, die from their disease within a few months.” A dozen STS surgeons put their names to a similar statement assuming zero 5-year survival for patients with lung metastases from CRC. This sets the scene for all survival beyond a few months being attributable to treatment, but neither paper provides adequate justification for the belief. An analysis of survival of 1,981 people with sarcoma in the Thames Cancer Registry from 1985 through 1994 shows survival to be much higher than MSK thoracic surgeons assumed (47). In the absence of control data the attribution of survival to lung operations is at best overstated and misleading.
The 34 operated patients collected over six years were reduced to 22 by retrospectively applied selection. To their credit MSK authors provided very long-term follow-up of surviving patients (48,49). “Four of six 10-year survivors survived more than 19 years even with multiple metastases and as many as nine thoracotomies”. This is a tiny sample. Rather than being proof of effective and generally applicable operative treatment these four cases should be seen as exceptional examples of indolent cancers in resilient patients.
Papers making the case for repeated pulmonary metastasectomy accept that there are no randomised controlled trials, no evaluation of the denominator and large heterogeneity amongst the sarcoma subtypes. This makes comparisons between metastasectomy with standard treatment and standard treatment alone difficult. What has changed is the multidisciplinary nature of cancer treatment and the individualised approach to care. It does seem from the available literature that the decision to operate on pulmonary metastases depends on a number of factors but primarily on the tumour behaviour. Slow growing tumours with fewer metastases that occur after the initial resection of the primary adjuvant treat and a disease-free interval before detection of the metastasis(es). It is hardly a surprise that this group do well whereas those outside these criteria (rapid growth, short disease-free interval or metastasis development during treatment or at presentation) fare less well. There may be consensus statements and national/international guidelines but the message is still the same. The disease is rare, it takes many forms and treatment is on an individual basis and determined by a multidisciplinary group. It is likely that the patients who have pulmonary metastasectomy are the ones that will, on account of the tumour behaviour, do well following metastasectomy and therefore have a prolonged survival.
Lung metastasectomy in germ cell tumours (GCT)
GCT are the most common malignancy in men between the ages of 15 and 44 years. Cisplatin-based chemotherapy regimens have been particularly successful for treatment but 10–20% of patients with lung metastases have a pulmonary metastasectomy and/or mediastinal lymph node excision. Metastasectomy has been included in treatment since the 1980s.
No RCTs have been performed in metastatic GCT, justified by the difficulty in running large prospective clinical trials when there are few cases. As a consequence, the surgical strategy and decision making depend on retrospective reviews. In all circumstances where lung metastasectomy is considered, the decision is based on the individual patient, the behaviour of the tumour and the prospect of long-term survival. In GCT with raised serum markers (alphafetoprotein) metastasectomy is used mainly to assess treatment response alongside decrease in the serum markers. Metastases from teratoma following treatment can show maturation (a benign state), involution or response failure with active malignant cells. It does appear that removal of active disease along with a corresponding fall in serum markers is indicative of disease control and therefore increased survival. In patients with residual intrathoracic disease after completion of chemotherapy. Survival rates approach 90% at 5 years in some studies. Metastasectomy is ideally performed shortly after completion of chemotherapy, with an appropriate window of approximately 4 weeks for patient optimization. This reduces unnecessary chemotherapy.
In summary, pulmonary metastasectomy for patients with GCTs is associated with better survival than for almost all other primary tumour histologies. The nature of the disease and small patient populations means that most of the data available is extrapolated from the general metastasectomy population or based on retrospective clinical reviews. Large, randomized control trials would be needed to determine best practice for this patient population but this is unlikely.
Lung metastasectomy in trophoblastic tumours
Trophoblastic tumours are unique in that the cells are from the offspring of the mother rather than being her own. Often there is a long disease-free interval between the molar pregnancy and presentation and the prognosis is better in patients without thoracic disease. The presence of metastases does not preclude treatment but changes in the character of the metastases after chemotherapy suggests that chemoresistance is occurring and metastasectomy may direct treatment if active tumour cells are found within the specimens. The number of cases that present each year is small and the aim of treatment is not to eradicate all disease but to resect the growing nodule(s) and determine if the chemotherapy has worked. The presence of a raised serum HCG and a growing nodule indicates active disease and the nodule removal along with a fall in serum HCG would indicate successful disease control. A search of PubMed found one analysis of a series of 97 cases published in 1988. Few had lung only metastases and the only conclusion we can offer is, at present, systemic treatment with resection of metastases helps treatment decisions (50).
For both germ cell and trophoblastic tumours increasingly targeted systemic treatments are the mainstay with the surgeon asked to resect selectively and conservatively to assess treatment response and to provide tissue to guide further treatment.
What might be the way ahead for metastatic carcinoma in the lung?
The available evidence from RCTs and their meta-analysis shows no survival advantage from earlier detection of asymptomatic lung metastases. There is evidence of a small loss of survival which is in line with the risks of treatments without benefit. A rational society with an eye on soaring health cost would seek to conserve health care funds to be spent to improve the health of its members.
It would seem obvious that claims for clinical benefit based on self-proclaimed effectiveness in uncontrolled follow-up studies should be more carefully scrutinised. The proliferation of specialist journals allows for the publication of papers written by those providing treatments, reviewed and edited by like-minded interventionalists, and cited as evidence by those who want to promote their treatments in a medical marketplace.
Repeated investigations have a net negative impact on mental health causing what patients call “scanxiety”. The phrase “to give reassurance” is no better than a marketing ploy. If patients informed of the facts seek screening, they can be investigated on an individual basis after being counselled. It suggests that they may be better off not knowing.
Lung metastases are in nearly all cases a component of blood-borne systemic cancer and should be treated in that context. As they are, in nearly all cases, the most easily imaged component, patients would be better served if their metastases were allowed to remain so that treatment response or failure can be easily and cost effectively monitored.
As with the introduction of new systemic therapies, all surgical and local interventional treatments should be considered for prospective controlled studies, expertly and impartially designed. The argument that patients will not want to be in trials is greatly overplayed. When treatments were needed for Covid-19 large trials were run at record speed with sufficiently large number of willing participants. Public motives for accepting randomisation are a mix of altruism and wanting to be first to have access to what might prove to be the better treatment. Both are understandable.
Conclusions
The only disease for which there has been a careful analysis up to including an RCT is CRC. This contradicts the widespread belief that resection of asymptomatic lung metastases for local treatment offers true benefit in the large majority of cases. It does not preclude the occasional freakish occurrence of a truly solitary lung metastasis, the removal of which renders the patient cancer free. We believe that it is implausible that there is a different “truth” for other carcinomas.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Nardini and David A. Waller) for the series “Local Treatment of Pulmonary Metastasis” published in AME Surgical Journal. The article has undergone external peer review.
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-23-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-23-9/coif). The series “Local Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. PulMiCC was funded by Cancer Research UK (No. C7678/A11393). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Handy JR, Bremner RM, Crocenzi TS, et al. Expert Consensus Document on Pulmonary Metastasectomy. Ann Thorac Surg 2019;107:631-49. [Crossref] [PubMed]
- van Dorp M, Gonzalez M, Daddi N, et al. Metastasectomy for colorectal pulmonary metastases: a survey among members of the European Society of Thoracic Surgeons. Interdiscip Cardiovasc Thorac Surg 2023;36:ivad002.
- Treasure T. Dr Samways writes to the Editor. Newcastle: Cambridge Scholars; 2021:281.
- Medical Research Council. Streptomycin treatment of pulmonary tuberculosis: a report of the streptomycin in tuberculosis trials committee. BMJ 1948;ii:769-82.
- Allbutt TC. A discussion on the remedial treatment of tuberculosis I. BMJ 1899;ii:1149-54.
- Samways DW. Ocean voyages in pulmonary phthisis. BMJ 1899;ii:1817. [Crossref]
- Treasure T, Williams N. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials (electronic response). BMJ 2016. Available online: http://www.bmj.com/content/327/7429/1459/rapid-responses
- Barr J. A Clinical Lecture on the Treatment of Serous Effusions. BMJ 1904;i:649-52. [Crossref]
- Barr J. Pleural effusion and its treatment. Lancet 1907;ii:1787-8. [Crossref] [PubMed]
- Treasure T. 20.This Correspondence Must Now Cease 1906-7. Dr Samways writes to The Editor. Newcastle: Cambridge Scholars; 2021:131-6.
- Simpson G. Pleural effusion and its treatment. Lancet 1907;ii:1567-8. [Crossref]
- Treasure T, Hollman A. The surgery of mitral stenosis 1898-1948: why did it take 50 years to establish mitral valvotomy? Ann R Coll Surg Engl 1995;77:145-51. [PubMed]
- Samways D. Cardiac peristalsis: its nature and effects. Lancet 1898;i:927. [Crossref]
- Samways DW. The Left Auricle in Mitral Stenosis: Hypertrophy and Dilatation. Br Med J 1896;2:1567-8. [Crossref] [PubMed]
- Treasure T. The Heart Club. 1st ed. London: Clink Street; 2017.
- Treasure T. Documenting the dramatic effects of operative treatment of mitral stenosis. J R Soc Med 2017;110:411-3. [Crossref] [PubMed]
- Treasure T. 25. Bloodletting. Dr Samways writes to The Editor. Newcastle: Cambridge Scholars; 2021:169-77.
- Treasure T, Farewell V, Macbeth F, et al. Pulmonary Metastasectomy versus Continued Active Monitoring in Colorectal Cancer (PulMiCC): a multicentre randomised clinical trial. Trials 2019;20:718. [Crossref] [PubMed]
- Milosevic M, Edwards J, Tsang D, et al. Pulmonary Metastasectomy in Colorectal Cancer: updated analysis of 93 randomized patients - control survival is much better than previously assumed. Colorectal Dis 2020;22:1314-24. [Crossref] [PubMed]
- Brew-Graves C, Farewell V, Monson K, et al. Pulmonary metastasectomy in colorectal cancer: health utility scores by EQ-5D-3L in a randomized controlled trial show no benefit from lung metastasectomy. Colorectal Dis 2021;23:200-5. [Crossref] [PubMed]
- Treasure T, Farewell V, Macbeth F, et al. The Pulmonary Metastasectomy in Colorectal Cancer cohort study: Analysis of case selection, risk factors and survival in a prospective observational study of 512 patients. Colorectal Dis 2021;23:1793-803. [Crossref] [PubMed]
- Treasure T, Farewell V, Macbeth F, et al. The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) burden of care study: Analysis of local treatments for lung metastases and systemic chemotherapy in 220 patients in the PulMiCC cohort. Colorectal Dis 2021;23:2911-22. [Crossref] [PubMed]
- Williams NR, Patrick H, Fiorentino F, et al. Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) randomised controlled trial: a systematic review of published responses. Eur J Cardiothorac Surg 2022;62:ezac253. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Siebenhüner AR, Güller U, Warschkow R. Population-based SEER analysis of survival in colorectal cancer patients with or without resection of lung and liver metastases. BMC Cancer 2020;20:246. [Crossref] [PubMed]
- Schirren J, Schirren M, Lampl L, et al. Surgery for pulmonary metastases: quo vadis? Eur J Cardiothorac Surg 2017;51:408-10. [Crossref] [PubMed]
- Wade TP, Virgo KS, Li MJ, et al. Outcomes after detection of metastatic carcinoma of the colon and rectum in a national hospital system. J Am Coll Surg 1996;182:353-61. [PubMed]
- Fiorentino F, Hunt I, Teoh K, et al. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med 2010;103:60-6. [Crossref] [PubMed]
- Fenton HM, Finan PJ, Milton R, et al. National variation in pulmonary metastasectomy for colorectal cancer. Colorectal Dis 2021;23:1306-16. [Crossref] [PubMed]
- McCormack PM, Burt ME, Bains MS, et al. Lung resection for colorectal metastases. 10-year results. Arch Surg 1992;127:1403-6. [Crossref] [PubMed]
- Okumura S, Kondo H, Tsuboi M, et al. Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg 1996;112:867-74. [Crossref] [PubMed]
- Utley M, Treasure T. Interpreting data from surgical follow-up studies: the role of modeling. J Thorac Oncol 2010;5:S200-2. [Crossref] [PubMed]
- Utley M, Treasure T, Linklater K, et al. Better out than in? The resection of pulmonary metastases from colorectal tumours. In: Xie X, Lorca F, Marcon E, editors. Operations Research for Health Care Engineering: Proceedings of the 33rd International Conference on Operational Research Applied to Health Services. Saint-Etienne: Publications de l'Universitaire de Saint-Etienne; 2008:493-500.
- Northover J, Houghton J, Lennon T. CEA to detect recurrence of colon cancer. JAMA 1994;272:31. [Crossref] [PubMed]
- Treasure T, Monson K, Fiorentino F, et al. Operating to remove recurrent colorectal cancer: have we got it right? BMJ 2014;348:g2085. [Crossref] [PubMed]
- Treasure T, Monson K, Fiorentino F, et al. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ Open 2014;4:e004385. [Crossref] [PubMed]
- Godlee F. Colorectal cancer: a cautionary tale. BMJ 2014;348: [Crossref]
- Mokhles S, Macbeth F, Farewell V, et al. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg 2016;103:1259-68. [Crossref] [PubMed]
- Jeffery M, Hickey BE, Hider PN, et al. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2016;11:CD002200. [Crossref] [PubMed]
- Rosenberg C, Golden J. Framing Disease: Studies in cultural history. New Brunswick, New Jersey: Rutgers University Press; 1992.
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Koch R. Die aetiologie der Tuberkulose. Berliner Klinische Wochenschrift 1882;19:221-3.
- Sakula A. Robert Koch: centenary of the discovery of the tubercle bacillus, 1882. Thorax 1982;37:246-51. [Crossref] [PubMed]
- Treasure T. Oligometastatic cancer: an entity, a useful concept, or a therapeutic opportunity? J R Soc Med 2012;105:242-6. [Crossref] [PubMed]
- Van Raemdonck D, Treasure T, Van Cutsem E, et al. Pulmonary Metastasectomy in Colorectal Cancer: has the randomized controlled trial brought enough reliable evidence to convince believers in metastasectomy to reconsider their oncological practice? Eur J Cardiothorac Surg 2021;59:517-21. [Crossref] [PubMed]
- Martini N, Huvos AG, Miké V, et al. Multiple pulmonary resections in the treatment of osteogenic sarcoma. Ann Thorac Surg 1971;12:271-80. [Crossref] [PubMed]
- Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736. [Crossref] [PubMed]
- Beattie EJ Jr. Surgical treatment of pulmonary metastases. Cancer 1984;54:2729-31. [Crossref] [PubMed]
- Beattie EJ, Harvey JC, Marcove R, et al. Results of multiple pulmonary resections for metastatic osteogenic sarcoma after two decades. J Surg Oncol 1991;46:154-5. [Crossref] [PubMed]
- Kumar J, Ilancheran A, Ratnam SS. Pulmonary metastases in gestational trophoblastic disease: a review of 97 cases. Br J Obstet Gynaecol 1988;95:70-4. [Crossref] [PubMed]
Cite this article as: Treasure T, Anderson J, Macbeth F. A perspective on lung metastasectomy: a review of a flawed concept and the failure to use available evidence resulting in an illusion of benefit. AME Surg J 2023;3:34.


