
Periarticular dextrose prolotherapy after total knee arthroplasty: a retrospective case series
Introduction
Total knee arthroplasty (TKA) is one of the most frequently performed orthopedic procedures in the United States. It is most commonly used for patients with end-stage osteoarthritis (OA) or inflammatory conditions of the knee. Patients routinely report significant levels of improvement in pain and quality of life after the procedure (1-3), yet a significant number of patients continue to experience knee pain even after undergoing TKA (4-6). These continued symptoms are considered to be multifactorial, and may be attributed to a number of different biological, surgical, and psychological factors (5,7,8). Conservative post-op care includes but is not limited to physical therapy, bracing, oral and topical analgesics, transcutaneous electrical nerve stimulation (TENS) units, and acupuncture. For refractory pain, genicular nerve ablation and selective peripheral nerve resection can also be performed (9-11). These procedures require further training, may be technically challenging, are invasive, and usually performed in a surgical setting needing much auxiliary assistance. Geniculate nerve blocks followed by radiofrequency ablation has been a more sought-after procedure in recent years but patient access may be limited due to various factors including: cost, insurance coverage, and limited number of specialists able to perform the procedure. Additionally, Klement et al. wrote of the efficacy of intra-articular corticosteroid injection after TKA in certain patients (12). This may be considered less invasive, however, may carry an increased risk of joint infection.
In this retrospective analysis of case series, we present a novel, easy, and relatively non-invasive approach towards idiopathic post-TKA pain using periarticular prolotherapy injections. The analgesic effect of periarticular injection after TKA has been well documented (13). Though its use post-TKA has not been reported in the literature, the efficacy of prolotherapy in the treatment of knee OA has been demonstrated in numerous prior studies (14,15). In addition, there are few absolute contraindications to prolotherapy, the primary of which is acute infections of the involved area such as cellulitis, local abscess, or septic arthritis, similar to any injection type (16).
Traditional prolotherapy vs. neural prolotherapy (NPT)
Traditional dextrose based prolotherapy typically involves injection of a hypertonic dextrose into damaged joints, ligaments, or tendons, and it is postulated to promote cell proliferation and functional restoration in the affected area. The term Prolotherapy was first coined by Hackett in the 1950s (17). He, along with his student Hemwall, expanded the concept and became pioneers in the field incorporating the Hackett-Hemwall Technique which involves multiple injections along the enthesis of the involved joint (Figure 1A).
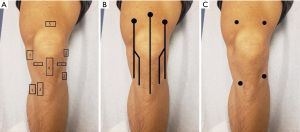
Conversely, perineural injection therapy (PIT) is a more recent advancement in regenerative medicine which targets cutaneous nerves as a potential pain generator. First described by Pybus and Wyburn-Mason, PIT targets neurogenic inflammation in subcutaneous nerves that potentially generates pain (18,19). This similar concept was documented by Hackett who wrote “inflammation neuritis and other antidromic impulses are transmitted to blood vessels in nerves and surrounding tissues stimulating a release of excess neurohumoral mediator substance which cause a neurovascular vasodilation-edema-sterile inflammation neuritis” (20). Lyftgoft further refined PIT using 5% dextrose treating Achilles tendinopathy, as well as knee, shoulder, elbow, and low back pain with subcutaneous prolotherapy which later became known as NPT (21-23). NPT injections are given along sensory nerves at their points of fascial penetration where they reach the subcutaneous plane (24). The cutaneous nerves are palpated along its course until tender chronic constrictive injury (CCI) points are encountered in which a series of injections are given at various points along the nerve (Figure 1B). It is thought that dextrose binds to presynaptic calcium channels, inhibiting the release of neurodegenerative peptides, thus decreasing neurogenic inflammation. This is postulated to result in pain reduction, regression of soft tissue swelling, and relief of CCI constrictions, restoring normal nerve growth factor flow, facilitating nerve repair (25).
Rezasoltani et al. showed that subcutaneous periarticular prolotherapy injections at four locations (where the periarticular nerves exit the joint capsule) had comparable effects to intra-articular prolotherapy injections with regards to pain and disability due to knee OA (24). This differed from the NPT technique which involves injections along superficial nerves and CCI constrictions. Rezasoltani’s methods served as our guide to evaluate the effects of periarticular prolotherapy injections on patients with post-TKA pain (Figure 1C). Typically, prolotherapy injections are performed serially (which may be every month for 3–4 months) until the maximal or desired benefit is achieved. We present the following cases in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-38/rc).
Case presentation
Injection technique
A solution consisting of 2 mL 50% dextrose, 2 mL normal saline, and 2 mL 1% lidocaine was used and injected subcutaneously into 4 points in the periarticular area similar to Rezasoltani et al. (24). However, we did not perform the injection over the fibular head to avoid the common peroneal nerve and instead injected 1 cm anterior to it. Also, our total volume was less using 6 vs. 10 mL. Approximately 1.5 mL of solution was injected in a fanning motion superiorly in 3 directions withdrawing the needle just below the skin and reinserting in a different direction without removing the tip from the initial puncture site at each point. If there is improvement with the initial injections, the procedure can be repeated serially in monthly intervals until either the maximal or desired benefit is achieved.
Patient selection criteria
The patients were selected based on the following requirements. They all presented with knee pain which was deemed to be stemming from the joint or immediately surrounding tissues. The pain could not be referred from another joint (e.g., hip or spine). They must have undergone TKA at least 1 year prior on the affected knee without evidence of hardware failure, fracture, or other findings on X-ray which would have otherwise explained their pain. No joint laxity was evaluated on exam. They had all failed standard conservative treatments such as medications, bracing and therapeutic exercise. They did not have active infections and were deemed safe to receive prolotherapy injections. Patients were selected consecutively between February 2019 to July 2019 and all came from a single Veteran Affairs hospital. Patients had in-person encounters for their evaluations, injections and follow-up. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
A male in his 70s presented with chronic left knee pain. His symptoms began after bilateral TKA 10 years prior. He reported constant pain rated 3–4/10 on the Numeric Pain Rating Scale (NPRS) in severity on average. He underwent arthroscopic lysis of adhesions of the left knee 4 years prior with no improvement. Oral (acetaminophen 500 mg q6 hours) and topical analgesics (diclofenac 1% 1 g q8 hours, lidocaine 4% patch q24 hours) were used for several months with minimal improvement. Pre-injection WOMAC index score was 65 (scale 0–96, lower score is better) (26). He received a series of two left knee periarticular prolotherapy injections 6 weeks apart with excellent relief, bringing his average pain level to 1–2/10 and WOMAC index score to 41 at 1-week follow-up, which was sustained at 8-month follow-up. He states to walking a mile per day and no longer limits his distance due to pain.
Case 2
Another male in his 70s presented with chronic left knee pain after TKA 9 months prior. He reported constant pain rated 5–6/10 in severity on average, worse with jogging and using stairs. Pre-injection WOMAC index score was 49. Previous treatments did not provide significant improvement which included several months of topical menthol cream, topical NSAIDS (diclofenac 1% gel q8 hours) and aqua therapy. The patient was given left knee periarticular prolotherapy injections with complete and immediate relief of pain. At the 7.5-month follow-up he reported continued relief and with pain rated 0/10 at rest and with light activities such as walking. WOMAC index score improved to 35. He still reported pain when using stairs. He reported an overall increase in ability to jog and has been able to progress to sprinting, which he was previously unable to do.
Case 3
A female in her 60s with a history of a left TKA 6 years prior presented with chronic left knee pain after a fall onto the joint 2 months prior. She reported constant pain rated 6/10 in severity on average. Pre-injection WOMAC index score was 59. She reported increased pain in the mornings and with ambulation. Previous therapies included naproxen (200–400 mg q8–12 hours) and neoprene knee sleeve for several months with minimal relief. She was given a series of two left knee periarticular prolotherapy injections. She reported immediate decrease in pain to 4/10 after first injection, with lasting relief for 4 months. She was given another with reported complete and immediate resolution of pain lasting 3 months until she sustained a mechanical fall onto her left knee. At the 11-month follow-up her pain was 2/10, which she noted was still improved from prior to the injections. WOMAC index score improved to 25. She continued to have pain with ascending and descending stairs but is less severe and less functionally limiting. She has been using oral and topical analgesics in the interim but does not feel they have been as helpful as the prolotherapy.
Discussion
TKA is generally considered an effective procedure for patients with end stage OA or inflammatory conditions of the knee. However, a significant number of patients experience continued pain post-op (6). Currently, there are limited treatment options available. Such treatments include intra-articular injections, intrathecal morphine, femoral nerve blocks, and genicular nerve ablation which may be invasive and pose potential risk for infection (26,27). Less invasive options such as medications may not provide adequate relief (27). Additionally, these procedures require additional training and are performed in a surgical type setting which may make it less accessible to patients in areas where there are limited amount of providers who perform them or limited by cost. Here, we present a less invasive treatment for post-TKA pain using a novel periarticular prolotherapy injection technique which is easy to learn and perform. The injection technique utilized only four injection sites which was significantly less versus typical dextrose prolotherapy and PIT techniques. This ultimately may prove more tolerable for the patient.
The lead author previously experimented this technique with a wide range of knee pathologies, and, while some patients had no to little relief, a number of patients demonstrated good relief. We decided to further investigate this technique in a more specific subset of patients (post-TKA knee pain), and these are the results we experienced thus far.
In our case series, no complications or adverse effects were reported, consistent with prior reports in the literature. In a systematic review on the safety of prolotherapy for the management of lower extremity pathology, Sanderson et al. found very few adverse events reported in the literature; the primary adverse event reported was local pain at the injection site. No incidence of infection was noted (28). This is partially due to the inherent nature of prolotherapy typically consisting of a relatively benign dextrose injection. The mechanism is still unclear, but we can give insight as to our analysis of it versus similar related methods. In the cases where the knee joint is unstable having laxity, prolotherapy can be a means to help fortify the surrounding stabilizing tendons by its traditional mechanism of promoting the local inflammation response to in turn re-stimulate the healing cascade. However, in these cases where there were no signs of instability, the mechanism appears to have more of an analgesic effect similar to a geniculate nerve block. One may argue the injection sites are located over the geniculate nerves and essentially an indirect nerve block is being performed, however, this cannot explain the months of relief that were experienced afterwards. These novel injections are more superficial and using very small volume of total solution with even less amount of anesthetic used in typical geniculate nerve blocks used prior to TKA. The latter are aimed to improve post operative recovery, physical performance scores, decreasing the need for opioids and recovery analgesics (e.g., 20 mL of anesthetic) (29,30). It also has similarities to the NPT pathway to alleviate neurogenic inflammation but at a higher dextrose concentration and using less injection sites versus typical NPT techniques. NPT typically involves the use of 5% dextrose to prevent an inflammatory reaction, however, in our cases 16% dextrose was used (Rezasoltani used a 10% dextrose solution) (24). This may be counter intuitive considering NPT involves decreasing neurogenic inflammation at a lower concentration whereas the concentration used here should technically cause an inflammation response as in traditional dextrose prolotherapy.
Each of our three patients experienced greater than 50% immediate and sustained relief in pain for several months allowing for an increase in function. While the mechanism is still unclear and needs further work to elucidate, perhaps we have demonstrated that despite this, higher concentrations of dextrose may have benefits to address neurogenic inflammation as well. Further research is warranted into the effectiveness of periarticular prolotherapy using this technique using variable concentrations.
Limitations
This case series is retrospective in nature and utilizes a small sample size of only 3 patients. It will be helpful to further study this technique in a randomized, prospective nature with a control arm.
Conclusions
This novel periarticular prolotherapy injection technique can be considered as a relatively safe and effective treatment option for chronic post-TKA pain. It may be easy to learn and perform making it ultimately more accessible to specialists and patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-38/rc
Peer Review File: Available at https://asj.amegroups.com/article/view/10.21037/asj-21-38/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neuprez A, Neuprez AH, Kaux JF, et al. Total joint replacement improves pain, functional quality of life, and health utilities in patients with late-stage knee and hip osteoarthritis for up to 5 years. Clin Rheumatol 2020;39:861-71. [Crossref] [PubMed]
- Skou ST, Roos EM, Laursen MB, et al. A Randomized, Controlled Trial of Total Knee Replacement. N Engl J Med 2015;373:1597-606. [Crossref] [PubMed]
- Canovas F, Dagneaux L. Quality of life after total knee arthroplasty. Orthop Traumatol Surg Res 2018;104:S41-6. [Crossref] [PubMed]
- Rice DA, Kluger MT, McNair PJ, et al. Persistent postoperative pain after total knee arthroplasty: a prospective cohort study of potential risk factors. Br J Anaesth 2018;121:804-12. [Crossref] [PubMed]
- Wylde V, Beswick A, Bruce J, et al. Chronic pain after total knee arthroplasty. EFORT Open Rev 2018;3:461-70. [Crossref] [PubMed]
- Beswick AD, Wylde V, Gooberman-Hill R, et al. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [Crossref] [PubMed]
- Thompson R, Novikov D, Cizmic Z, et al. Arthrofibrosis After Total Knee Arthroplasty: Pathophysiology, Diagnosis, and Management. Orthop Clin North Am 2019;50:269-79. [Crossref] [PubMed]
- Cheuy VA, Foran JRH, Paxton RJ, et al. Arthrofibrosis Associated With Total Knee Arthroplasty. J Arthroplasty 2017;32:2604-11. [Crossref] [PubMed]
- Chia SK, Wernecke GC, Harris IA, et al. Peri-articular steroid injection in total knee arthroplasty: a prospective, double blinded, randomized controlled trial. J Arthroplasty 2013;28:620-3. [Crossref] [PubMed]
- Erdem Y, Sir E. The Efficacy of Ultrasound-Guided Pulsed Radiofrequency of Genicular Nerves in the Treatment of Chronic Knee Pain Due to Severe Degenerative Disease or Previous Total Knee Arthroplasty. Med Sci Monit 2019;25:1857-63. [Crossref] [PubMed]
- Zhong G, Liang Z, Kan J, et al. Selective peripheral nerve resection for treatment of persistent pain around the knee joint after total knee arthroplasty. J Int Med Res 2018;46:2301-6. [Crossref] [PubMed]
- Klement MR, Luzzi AJ, Siddiqi A, et al. Intra-articular Corticosteroid Injection Following Total Knee Arthroplasty: Is It Effective? J Arthroplasty 2019;34:303-8. [Crossref] [PubMed]
- Kim TW, Park SJ, Lim SH, et al. Which analgesic mixture is appropriate for periarticular injection after total knee arthroplasty? Prospective, randomized, double-blind study. Knee Surg Sports Traumatol Arthrosc 2015;23:838-45. [Crossref] [PubMed]
- Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med 2013;11:229-37. [Crossref] [PubMed]
- Rahimzadeh P, Imani F, Faiz SHR, et al. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging 2018;13:73-9. [Crossref] [PubMed]
- Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care 2010;37:65-80. [Crossref] [PubMed]
- Hackett GS, Henderson DG. Joint stabilization; an experimental, histologic study with comments on the clinical application in ligament proliferation. Am J Surg 1955;89:968-73. [Crossref] [PubMed]
- Reeves KD, Lyftogt J. Prolotherapy: Regenerative Injection Therapy. In: Waldman SD. editor. Pain Management. 2nd edition. Philadelphia: Saunders (Elsevier), 2011:1027-44.
- Geppetti P, Nassini R, Materazzi S, et al. The concept of neurogenic inflammation. BJU Int 2008;101:2-6. [Crossref] [PubMed]
- Hackett GS. Uninhibited reversible antidromic vasodilation in pathophysiologic diseases: Arteriosclerosis, carcinogenesis, neuritis, and osteoporosis. Angiology 1966;17:109-18. [Crossref] [PubMed]
- Lyftogt J. Subcutaneous prolotherapy for Achilles tendinopathy: the best solution? Australasian Musculoskeletal Medicine 2007;12:107-9.
- Lyftogt J. Subcutaneous prolotherapy treatment of refractory knee, shoulder, and lateral elbow pain. Australasian Musculoskeletal Medicine 2007;12:110-2.
- Lyftogt J. Prolotherapy for Recalcitrant Lumbago. Australasian Musculoskeletal Medicine 2008;13:18-20.
- Rezasoltani Z, Taheri M, Mofrad MK, et al. Periarticular dextrose prolotherapy instead of intra-articular injection for pain and functional improvement in knee osteoarthritis. J Pain Res 2017;10:1179-87. [Crossref] [PubMed]
- Weglein AD. Neural Prolotherapy. Journal of Prolotherapy 2011;3:639-43.
- Roecker Z, Quinlan ND, Browne JA, et al. Risk of Periprosthetic Infection Following Intra-Articular Corticosteroid Injections After Total Knee Arthroplasty. J Arthroplasty 2020;35:1090-4. [Crossref] [PubMed]
- Karlsen AP, Wetterslev M, Hansen SE, et al. Postoperative pain treatment after total knee arthroplasty: A systematic review. PLoS One 2017;12:e0173107. [Crossref] [PubMed]
- Sanderson LM, Bryant A. Effectiveness and safety of prolotherapy injections for management of lower limb tendinopathy and fasciopathy: a systematic review. J Foot Ankle Res 2015;8:57. [Crossref] [PubMed]
- Li JW, Ma YS, Xiao LK. Postoperative Pain Management in Total Knee Arthroplasty. Orthop Surg 2019;11:755-61. [Crossref] [PubMed]
- Binici Bedir E, Kurtulmuş T, Başyiğit S, et al. A comparison of epidural analgesia and local infiltration analgesia methods in pain control following total knee arthroplasty. Acta Orthop Traumatol Turc 2014;48:73-9. [Crossref] [PubMed]
Cite this article as: Barawid EL, Davis SM, Ardestani A, Turchi RD. Periarticular dextrose prolotherapy after total knee arthroplasty: a retrospective case series. AME Surg J 2023;3:28.


