Hereditary gastric and breast cancer syndromes with lung metastasis: narrative review of molecular and clinical insights
Introduction
Hereditary diffuse gastric cancer (HDGC) is a rare cancer syndrome characterized by a high prevalence of diffuse gastric cancer (DGC) and invasive lobular breast cancer (LBC) (1,2).
This syndrome has been mainly related to germline variants in the CDH1 gene, located on the 16q22.1 chromosome (3). CDH1 germline mutation is known as a crucial target for cancer initiation, yet, by itself, not sufficient for invasive gastric cancer (GC) development (4). This gene encodes a cell-to-cell adhesion molecule, E-cadherin, where its loss is linked to increased infiltrative and metastatic potential (1). The loss of E-cadherin expression is associated with familial cancer syndrome, HDGC, DGC signet-ring cell type, LBC, and other breast cancers progression (5-11). The major feature of recurrence in GC is intra-abdominal spread with the most common site of metastasis of the liver (in 48% of metastatic cancer patients) (12). Among the metastatic sites, a relatively rare incidence of pulmonary metastasis could be present in GC patients (13,14). One of the major LBC metastatic sites indeed is the lung and overlap of these two entities in HGDC prompted us to analyze possible correlations between DGC and LBC (15). We present this article in accordance with the Narrative Review reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-22-9/rc).
Methods
We conducted literature research on the topic, including English articles published in the years 1996–2022, where all authors contributed equally reaching a final consensus before the article submission. The search strategy is described in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 24–28/01/2022 |
| Databases and other sources searched | PubMed, Wiley, Springer, AME Surgical Journal |
| Search terms used | Hereditary gastric cancer (HGC), hereditary diffuse gastric cancer (HGDC), hereditary breast cancer (HBC), CDH-1, E-cadherin, lung metastasis |
| Timeframe | 1996–2022 |
| Inclusion and exclusion criteria | Language restrictions: English only |
| Selection process | Selection process was conducted independently and consensus obtained by all authors before submission |
Discussion
Role of CDH1 in hereditary cancers and relationship with histological features
CDH1 encodes for E-cadherin, an epithelial marker, considered as a tumor suppressor which interacts with β-catenin as an effector of the WNT signaling pathway. Loss of CDH1 is linked to increased infiltrative and metastatic potential (15). The translocation of β-catenin also represses the expression of phosphatase and tensin homolog (PTEN) , which is a tumor suppressor. CDH1 loss has also been associated with epidermal growth factor receptor (EGFR) activation through pro-tumorigenic RAS/RAF/MEK, FAK/c-Src and PI3K/AKT/mTOR pathways, which enhance cell proliferation and motility (5-10). It is known that loss of E-cadherin (CDH1), Smad4, and p53 has an important role in breast and GCs formation (16). CDH1 hypermethylation is present in LBC and other breast cancer types, associated with reduced estrogen receptor (ER) and progesterone receptor (PR) expression, accompanied by lung, bone, brain and lymph node metastasis (15,17,18). Frequently, LBC and DGC patients carrying CDH1 germline mutations present similar histological appearance with highly dispersive infiltrative cancer cells and signet-ring cell components, often followed by loss of E-cadherin on immunohistochemical staining (19). In CDH1 mutation carriers, two patterns of signet ring cells distribution have been described: in situ (confined to the basal membrane) and pagetoid spread below the preserved epithelium (5,6,19,20).
CDH1 and hereditary cancers metastasis
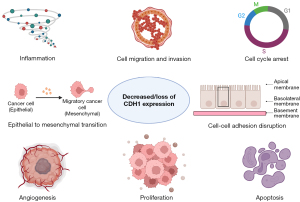
The loss of CDH1 is known to promote cancer metastasis by disrupting cell-cell adhesion and transcriptional changes induction (21). The role of E-cadherin in metastasis has been sought through a prism of epithelial to mesenchymal transition (EMT), which is regarded as an important event in various cancer metastases (7). The process of EMT, occurring both in physiological and pathological conditions may downregulate E-cadherin, promoting epithelial cells inhibition with fibroblast phenotype acquisition. These cells start being invasive, expressing Snail family transcription factors and suppressing E-cadherin (22). Molecularly, the loss of E-cadherin may happen due to germline mutations with transcription inhibitors overexpression—Snail, Slug, Twist, ZEB1, ZEB2 that are known to be related to tumor differentiation and metastasis, induced by TGF-β causing E-cadherin suppression (Figure 1) (7,23-25).
It is known that one of the common HGDC-syndrome metastatic sites is the lung (12). Several lines of evidence have described the metastatic pattern of intestinal and diffuse-type GC so far (26). On the whole, intestinal-type carcinomas more often metastasize to the liver and lungs and have a better prognosis compared to diffuse GC that metastasizes more frequently to the peritoneum and bones (26). A Dutch national cohort including 8,231 metastatic GC cases reported a 13% rate of lung metastases for intestinal and 7% for diffuse GC (P<0.0001) (26). Another Northern European series included 7,559 patients, with 5% of adenocarcinomas and 2% of signet ring carcinomas harboring lung metastases (12). A similar trend has been described in the Eastern series. Indeed, a Chinese cohort of 7,792 patients reported a 6.5% frequency of lung metastases for adenocarcinomas and 4.4% for signet ring cell cancers (14). Similarly, a large Korean cohort of 20,187 patients recorded a prevalence of lung metastases of 0.196% (193 patients). Most of these patients had a diagnosis of adenocarcinoma (71.4%), while only 16.6% of patients had a signet ring cell histology and 11.9% other rare histologies (13). Moreover, further confirmation of this data comes from a retrospective evaluation of the Surveillance, Epidemiology and End Result (SEER) database of the National Cancer Institute. A total of 1,104 patients with GC and lung metastases were identified, representing 6.02% of the cohort of patients with a new diagnosis of GC from 2010 to 2014. Of them, 21% had diffuse-type histology, while 72.6% had intestinal-type GC and the remaining portion had different and rare histologies (27).
Signet-ring cell GC metastasis to the lung has already been previously described in the literature (12,28). Biological differences between two GC histotypes may justify the different patterns of metastasis in GC. Firstly, loss of E-cadherin expression that is typical of diffuse GC was associated with increased recurrence to the peritoneum (P<0.01) and distant lymph nodes (P<0.01), though GC with liver metastases had relatively positive E-cadherin expression (29). Another biological evidence supporting the different patterns of metastasis is given by the different expressions of adhesion molecules. Adhesion of neoplastic cells to the mesothelium is favored by the presence of molecules such as CD44, whose expression is higher in poorly differentiated tumors such as signet ring cell carcinoma (30). Moreover, signet ring cell carcinomas produce mucin, which may infiltrate the surrounding stroma and help the tumor become invasive, thus facilitating spread to the serosa and peritoneum (12). Additionally, the proliferative activity in diffuse GC is increased in the deeper layers, resulting in a greater propensity for peritoneal seeding and metastasis to female reproductive organs (31,32).
Experimental models of CHD1 loss/alterations in DGS showed a diffuse morphology with signet-ring cells and lung metastasis with peritoneal carcinomatosis and it has confirmed that signet-ring cell morphology is overall more frequently observed in CDH1 mutations (33,34).
In breast cancer, lung metastases were specifically attributed to the triple-negative breast cancers (TNBC) phenotype, by activation of breast cancer stem cells via β-catenin/WNT signaling (35,36). One study demonstrated lung metastasis developing after 12 weeks of primary breast cancer, where both primary and metastatic tumor cells expressed E-cadherin and lacked Vimentin which indeed indicates no EMT occurrence (37). These data were supported by EMT markers panel assessment in 148 LBC cases, where only 2% demonstrated downregulation of E-cadherin and upregulation of mesenchymal markers (38). Additional findings suggest that E-cadherin is suppressing metastasis in invasive LBC (39). Signet-ring cell carcinoma was also the most common histologic pattern in TNBC and pathogenic variants of CDH1, detected in patients with DGC and resulted in extensive or metastatic disease in 24% of patients and its mutations are overall associated with distant metastases (34,40,41).
Given that loss of E-cadherin expression in carcinoma development is regarded as a sign of EMT and tumor progression, numerous studies aimed to investigate its pathways. Experimental loss of E-cadherin and Smad4 in cooperation display promotion of DGC development and metastatic progression. Knock-out of one CDH1 allele with both p53 and Smad4 alleles lead to lung metastasis of gastric carcinomas formation, suggesting that loss of E-cadherin and Smad4 expression promote lung metastasis through β-catenin activation (16), while activation of oncogenic KRAS accelerates this process. The transduction of normal gastric epithelial cells with KRAS had significantly decreased the expression of E-cadherin and increased the expression of Vimentin, which is another proof of EMT role in tumorigenesis and metastasis. Interestingly, KRAS inhibition in gastric adenocarcinomas led to the loss of infiltrative tumor border and fewer lung metastases (42). In addition to this data, there is also a report about the role of intermediate filaments KRT17, where their downregulation induces E-cadherin loss and EMT, leading to metastasis of DGC and worse prognosis (43).
The main issue nowadays is that the timeframe of HDGC and DGC progression to metastatic disease is yet to be studied, as most patients with DGC have advanced disease at the time of presentation and this emphasizes the importance of early identification of these patients to develop effective surveillance techniques (41).
CDH1 screening and management strategies
The patients with a family history of HDGC may use genetic testing for CDH1 mutations for individual risk assessment and consideration of prophylactic gastrectomy, while some studies confirm that all individuals with identified CDH1 mutation had a personal or family history of HDGC and shorter survival compared to those without abovementioned mutation (1-3,5,6,9,44,45). Increased availability and adoption of cancer gene panels have led to increased identification of variants of CDH1 gene mutations in patients with a family history of breast cancer (1). The patients with breast cancer history and confirmed CDH1 mutation found via genetic testing are at a higher risk of LBC and are at higher risk of DGC. However, there are numerous reports in patients with CDH1 mutations affecting exclusively LBC without any evidence of gastric tumors (46,47). Indeed the latest data demonstrate that carriers of CDH1 pathogenic or likely pathogenic variants and LBC have high rates of occult signet ring cell GC incidence (48).
The International Gastric Cancer Linkage Consortium (IGCLC) developed criteria to facilitate the screening of CDH1 mutation carriers, which have been proven to have excellent sensitivity and specificity (9). According to ultimate guidelines, the breast surveillance for HDCG starts at age of 30 with an annual magnetic resonance imaging (MRI) as a reporter of elevated risk of LBC incidence. If LBC is detected, CDH1 mutation carriers usually undergo breast-conserving surgery with reconstruction. Patients with DGC with ascertained CDH1 mutation are recommended to undertake total gastrectomy, where the latter could be also considered as a prophylactic procedure in the presence of known HGDC family history and confirmed CDH1 mutation (1,6,44,49). The oncogene role of CDH1 overexpression has been also demonstrated in lung adenocarcinoma, which can be a common site of metastasis in HGDC (50).
Conclusions
In conclusion, hereditary diffuse GC is a rare entity and, as opposed to LBC, lung metastases from this histologic subtype are very infrequent compared to intestinal GC. Many biological differences between signet ring and intestinal cancer cells explain the distinct pattern of metastasis for these two GC histological subtypes. Identification of a higher number of patients, affected by HGDC may somewhat shift our perception of this complex entity and provide a better understanding of the disease’s nature. There are still numerous questions to be answered in molecular and cellular events in CDH1 mutations carriers disease progression. A multidisciplinary approach in CDH1 mutations assessment is needed to accurately estimate its roles in HDGC and LBC familial syndromes, examining the risk for other cancers and conducting effective prevention and screening strategies with the probable establishment of susceptible genes testing panel (1,2,5,10,24,45,51).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Davide Tosi, Alessandro Palleschi and Paolo Mendogni) for the series “Management and Treatment of Lung Metastases” published in AME Surgical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-22-9/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-22-9/coif). The series “Management and Treatment of Lung Metastases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blair VR, McLeod M, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 2020;21:e386-97. [Crossref] [PubMed]
- Goud HK, Mehkari Z, Mohammed L, et al. Significance of E-cadherin Gene Mutations in Patients With Hereditary Diffuse Gastric Cancer Syndrome: A Systematic Review. Cureus 2020;12:e10406. [Crossref] [PubMed]
- Lo W, Zhu B, Sabesan A, et al. Associations of CDH1 germline variant location and cancer phenotype in families with hereditary diffuse gastric cancer (HDGC). J Med Genet 2019;56:370-9. [Crossref] [PubMed]
- Gamble LA, Heller T, Davis JL. Hereditary Diffuse Gastric Cancer Syndrome and the Role of CDH1: A Review. JAMA Surg 2021;156:387-92. [Crossref] [PubMed]
- Schrader KA, Masciari S, Boyd N, et al. Hereditary diffuse gastric cancer: association with lobular breast cancer. Fam Cancer 2008;7:73-82. [Crossref] [PubMed]
- Shenoy S. CDH1 (E-Cadherin) Mutation and Gastric Cancer: Genetics, Molecular Mechanisms and Guidelines for Management. Cancer Manag Res 2019;11:10477-86. [Crossref] [PubMed]
- Zhao H, Hu H, Chen B, et al. Overview on the Role of E-Cadherin in Gastric Cancer: Dysregulation and Clinical Implications. Front Mol Biosci 2021;8:689139. [Crossref] [PubMed]
- Breast Cancer Association Consortium. Breast Cancer Risk Genes - Association Analysis in More than 113,000 Women. N Engl J Med 2021;384:428-39. [Crossref] [PubMed]
- Luo W, Fedda F, Lynch P, et al. CDH1 Gene and Hereditary Diffuse Gastric Cancer Syndrome: Molecular and Histological Alterations and Implications for Diagnosis And Treatment. Front Pharmacol 2018;9:1421. [Crossref] [PubMed]
- Hu MN, Hu SH, Zhang XW, et al. Overview on new progress of hereditary diffuse gastric cancer with CDH1 variants. Tumori 2020;106:346-55. [Crossref] [PubMed]
- Han T, Jiang S, Zheng H, et al. Interplay between c-Src and the APC/C co-activator Cdh1 regulates mammary tumorigenesis. Nat Commun 2019;10:3716. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016;7:52307-16. [Crossref] [PubMed]
- Kong JH, Lee J, Yi CA, et al. Lung metastases in metastatic gastric cancer: pattern of lung metastases and clinical outcome. Gastric Cancer 2012;15:292-8. [Crossref] [PubMed]
- Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med 2018;7:3662-72. [Crossref] [PubMed]
- Liu J, Sun X, Qin S, et al. CDH1 promoter methylation correlates with decreased gene expression and poor prognosis in patients with breast cancer. Oncol Lett 2016;11:2635-43. [Crossref] [PubMed]
- Park JW, Jang SH, Park DM, et al. Cooperativity of E-cadherin and Smad4 loss to promote diffuse-type gastric adenocarcinoma and metastasis. Mol Cancer Res 2014;12:1088-99. [Crossref] [PubMed]
- Caldeira JR, Prando EC, Quevedo FC, et al. CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer 2006;6:48. [Crossref] [PubMed]
- Mehrotra J, Vali M, McVeigh M, et al. Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clin Cancer Res 2004;10:3104-9. [Crossref] [PubMed]
- Corso G, Figueiredo J, La Vecchia C, et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J Med Genet 2018;55:431-41. [Crossref] [PubMed]
- Taja-Chayeb L, Vidal-Millán S, Trejo-Becerril C, et al. Hereditary diffuse gastric cancer (HDGC). An overview. Clin Res Hepatol Gastroenterol 2021;46:101820. [Crossref] [PubMed]
- Liu X, Su L, Liu X. Loss of CDH1 up-regulates epidermal growth factor receptor via phosphorylation of YBX1 in non-small cell lung cancer cells. FEBS Lett 2013;587:3995-4000. [Crossref] [PubMed]
- Corso G, Figueiredo J, De Angelis SP, et al. E-cadherin deregulation in breast cancer. J Cell Mol Med 2020;24:5930-6. [Crossref] [PubMed]
- Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cell 2016;166:21-45. [Crossref] [PubMed]
- El Rami FE, Barsoumian HB, Khneizer GW. Hereditary diffuse gastric cancer therapeutic roadmap: current and novel approaches in a nutshell. Ther Adv Med Oncol 2020;12:1758835920967238. [Crossref] [PubMed]
- Yang Y, Zhao B, Lv L, et al. FBXL10 promotes EMT and metastasis of breast cancer cells via regulating the acetylation and transcriptional activity of SNAI1. Cell Death Discov 2021;7:328. [Crossref] [PubMed]
- Koemans WJ, Luijten JCHBM, van der Kaaij RT, et al. The metastatic pattern of intestinal and diffuse type gastric carcinoma - A Dutch national cohort study. Cancer Epidemiol 2020;69:101846. [Crossref] [PubMed]
- Sun Z, Liu H, Yu J, et al. Frequency and Prognosis of Pulmonary Metastases in Newly Diagnosed Gastric Cancer. Front Oncol 2019;9:671. [Crossref] [PubMed]
- Abe Y, Suzuki M, Tsuji K, et al. Lung metastasis from gastric cancer presenting as diffuse ground-glass opacities. Respir Med Case Rep 2020;30:101104. [Crossref] [PubMed]
- Shino Y, Watanabe A, Yamada Y, et al. Clinicopathologic evaluation of immunohistochemical E-cadherin expression in human gastric carcinomas. Cancer 1995;76:2193-201. [Crossref] [PubMed]
- Averbach AM, Jacquet P. Strategies to decrease the incidence of intra-abdominal recurrence in resectable gastric cancer. Br J Surg 1996;83:726-33. [Crossref] [PubMed]
- Marrelli D, Roviello F, de Manzoni G, et al. Different patterns of recurrence in gastric cancer depending on Lauren's histological type: longitudinal study. World J Surg 2002;26:1160-5. [Crossref] [PubMed]
- Verstegen MH, Harker M, van de Water C, et al. Metastatic pattern in esophageal and gastric cancer: Influenced by site and histology. World J Gastroenterol 2020;26:6037-46. [Crossref] [PubMed]
- Seidlitz T, Chen YT, Uhlemann H, et al. Mouse Models of Human Gastric Cancer Subtypes With Stomach-Specific CreERT2-Mediated Pathway Alterations. Gastroenterology 2019;157:1599-1614.e2. [Crossref] [PubMed]
- Nemtsova MV, Kalinkin AI, Kuznetsova EB, et al. Clinical relevance of somatic mutations in main driver genes detected in gastric cancer patients by next-generation DNA sequencing. Sci Rep 2020;10:504. [Crossref] [PubMed]
- Jin L, Han B, Siegel E, et al. Breast cancer lung metastasis: Molecular biology and therapeutic implications. Cancer Biol Ther 2018;19:858-68. [Crossref] [PubMed]
- Cha S, Lee E, Won HH. Comprehensive characterization of distinct genetic alterations in metastatic breast cancer across various metastatic sites. NPJ Breast Cancer 2021;7:93. [Crossref] [PubMed]
- Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015;527:472-6. [Crossref] [PubMed]
- McCart Reed AE, Kutasovic JR, Vargas AC, et al. An epithelial to mesenchymal transition programme does not usually drive the phenotype of invasive lobular carcinomas. J Pathol 2016;238:489-94. [Crossref] [PubMed]
- Padmanaban V, Krol I, Suhail Y, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature 2019;573:439-44. [Crossref] [PubMed]
- Marwitz T, Hüneburg R, Spier I, et al. Hereditary Diffuse Gastric Cancer: A Comparative Cohort Study According to Pathogenic Variant Status. Cancers (Basel) 2020;12:3726. [Crossref] [PubMed]
- Jacobs MF, Dust H, Koeppe E, et al. Outcomes of Endoscopic Surveillance in Individuals With Genetic Predisposition to Hereditary Diffuse Gastric Cancer. Gastroenterology 2019;157:87-96. [Crossref] [PubMed]
- Yoon C, Till J, Cho SJ, et al. KRAS Activation in Gastric Adenocarcinoma Stimulates Epithelial-to-Mesenchymal Transition to Cancer Stem-Like Cells and Promotes Metastasis. Mol Cancer Res 2019;17:1945-57. [Crossref] [PubMed]
- Li M, Rao X, Cui Y, et al. The keratin 17/YAP/IL6 axis contributes to E-cadherin loss and aggressiveness of diffuse gastric cancer. Oncogene 2022;41:770-81. [Crossref] [PubMed]
- van der Post RS, Vogelaar IP, Manders P, et al. Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1. Gastroenterology 2015;149:897-906.e19. [Crossref] [PubMed]
- Hansford S, Kaurah P, Li-Chang H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol 2015;1:23-32. [Crossref] [PubMed]
- Corso G, Intra M, Trentin C, et al. CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer 2016;15:215-9. [Crossref] [PubMed]
- Figueiredo J, Melo S, Carneiro P, et al. Clinical spectrum and pleiotropic nature of CDH1 germline mutations. J Med Genet 2019;56:199-208. [Crossref] [PubMed]
- Gamble LA, Rossi A, Fasaye GA, et al. Association Between Hereditary Lobular Breast Cancer Due to CDH1 Variants and Gastric Cancer Risk. JAMA Surg 2022;157:18-22. [Crossref] [PubMed]
- Slavin TP, Weitzel JN, Neuhausen SL, et al. Genetics of gastric cancer: what do we know about the genetic risks? Transl Gastroenterol Hepatol 2019;4:55. [Crossref] [PubMed]
- Ye T, Li J, Sun Z, et al. Cdh1 functions as an oncogene by inducing self-renewal of lung cancer stem-like cells via oncogenic pathways. Int J Biol Sci 2020;16:447-59. [Crossref] [PubMed]
- Massari G, Magnoni F, Favia G, et al. Frequency of CDH1 Germline Mutations in Non-Gastric Cancers. Cancers (Basel) 2021;13:2321. [Crossref] [PubMed]
Cite this article as: Ivanova M, Evangelista J, Venetis K, Sajjadi E, Lococo F, Corso G, Ghidini M, Fusco N. Hereditary gastric and breast cancer syndromes with lung metastasis: narrative review of molecular and clinical insights. AME Surg J 2023;3:39.