
Autologous breast reconstruction in a patient with thalidomide embryopathy: case report
Introduction
Thalidomide, also known as Contergan, has caused thalidomide embryopathy (TE) in more than 10,000 live-born children between 1957 and 1962 (1). Thalidomide was released in the late 1950’s as sedative and effective anti-emetic. It was especially used to treat morning-sickness in pregnant women until it was discovered to cause severe birth defects (2).
Phocomelia of the upper limb is the most striking deformity caused by thalidomide, and remains the stereotypical image of TE. However, several other abnormalities such as eye or ear malformations and internal organ defects are associated with thalidomide (2). By 2001, it was estimated that there were 5,000 survivors from the 10,000 babies born with TE (3). Nowadays, thalidomide survivors entered middle age, an age that is susceptible to breast cancer in women.
The authors present the first case of a patient with thalidomide induced phocomelia receiving breast reconstruction after multicentric breast cancer, in accordance with the CARE reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-88/rc).
Case presentation
A 49-year-old woman with a typical thalidomide induced phocomelia of the upper limbs presented with a multicentric breast cancer of her right breast. She had no organ anomalies, suffered neither deafness nor blindness. Her medical history was significant for clavicle fractures, that had to be operated and a benign breast lesion 30 years ago. She took no medication on a regular basis. Regarding her social history, she is married since 34 years and lives with her husband and their two children. She is unable to work and receives disability pension.
Breast cancer was diagnosed at the age of 49. The diagnostic assessment revealed two inhomogenous lesions of 1.2 cm × 0.9 cm × 0.8 cm on magnetic resonance imaging. Histological examination confirmed a multicentric moderately differentiated invasive ductal carcinoma. Immunohistochemical exam revealed estrogen receptor and progesterone receptor positive >95% and human epidermal growth factor 2 negative. Abdominal sonography, chest X-ray and whole-body bone scintigraphy were normal.
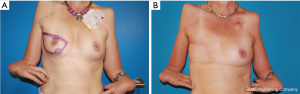
After multi-disciplinary evaluation, the patient received neoadjuvant chemotherapy with four cycles epirubicin/cyclophosphamide and four cycles docetaxel, followed by mastectomy and right axillary sentinel lymph node biopsy (Figure 1A,1B). The histopathological examination confirmed a complete tumor excision without infiltration of lymph and blood vessels.
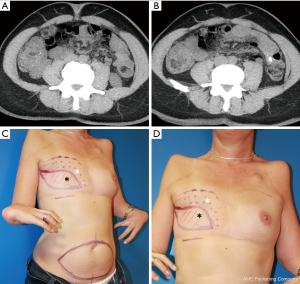
One year after mastectomy, we performed immediate-delayed autologous breast reconstruction using a deep inferior epigastric artery perforator flap (DIEP) as described by us previously (4). A computed tomography with angiography showed lateral and medial perforators of good caliber (Figure 2A,2B). The flap was based on two medial row perforators and anastomosed to the internal mammary vessels as usual. The flap was only de-epithelialized at its cranial part and the new inframammary crease was fixed onto the pectoral fascia in order to create a breast that fits the other side (Figure 2C,2D). The postoperative course was uneventful with sufficient flap vascularization and normal wound healing.
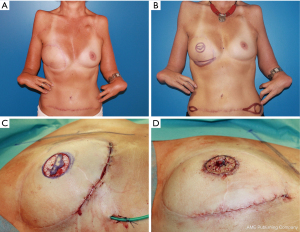
During the further course, corrections of the inframammary fold and the lateral parts of the abdominal scar were performed (Figure 3A,3B). The nipple areola complex (NAC) was reconstructed by a skate flap combined with a split-thickness skin graft that was taken from the abdomen (Figure 3C,3D).
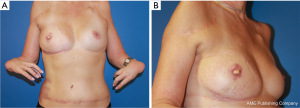
Currently, the patient is 60 years old and has been followed for 11 years now. She still reports to be very satisfied with her reconstructed breast and had no tumor recurrence (Figure 4A,4B).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We report about a rare case of successful breast reconstruction using a DIEP flap in a patient with TE. Although literature is limited, there is growing evidence that thalidomide survivors are increasingly susceptible to secondary health problems as they age (5,6). In 2015, a study assessed the prevalence of psychological co-morbidities in 193 affected patients and found that the lifetime prevalence of depressive disorders in that patient population was more than double that in the general population (7). Similar results were seen in a national study on health-related quality of life in which physical quality of life was significantly lower in TE individuals compared with the general Swedish population (8). In a more recent study, employment circumstances, health problems and health-related quality of life were assessed in 351 United Kingdom thalidomide survivors. Besides a wide range of secondary health problems, they found out that two fifths were unable to work. Most importantly, this study emphasizes the accumulative impact of disability over people’s lifetimes and highlights the value of a life course perspective when growing older with a disability (9).
Nowadays, most women survive breast cancer and must contend the long-term effects of mastectomy on quality of life and body image. Autologous breast reconstruction has shown to restore body image and improve health-related quality of life. Most importantly, several studies emphasize its impact on mental health with significantly increased psycho-social well-being after autologous breast reconstruction (10,11).
To the best of our knowledge there exists no literature on breast reconstruction in patients with TE or other physical impairments. In fact, patients with physical impairments are less likely to have breast conserving therapy and more likely to have a mastectomy without reconstruction compared to patients without disabilities (12). Our patient was known for a stable mental health and a good social background, which might have supported the decision to undergo breast reconstruction. Nevertheless, the accumulative impact of an absent breast in physically disabled patients needs to be considered individually.
In conclusion, we demonstrate that the DIEP flap was a feasible and safe technique to reconstruct the breast in our patient. Even though further studies and particularly an assessment of health-related quality of life would be necessary, we believe that breast reconstruction in physically disabled patients might be even more important in order to restore body image, improve quality of life and reduce the risk of mental disorders in that patient group.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-88/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-88/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ghassemi Jahani SA, Danielsson A, Karlsson J, et al. Middle-aged individuals with thalidomide embryopathy have undergone few surgical limb procedures and demonstrate a high degree of physical independence. PLoS One 2017;12:e0186388. [Crossref] [PubMed]
- Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today 2015;105:140-56. [Crossref] [PubMed]
- Bent N, Tennant A, Neumann V, et al. Living with thalidomide: health status and quality of life at 40 years. Prosthet Orthot Int 2007;31:147-56. [Crossref] [PubMed]
- Otte M, Nestle-Krämling C, Fertsch S, et al. Conservative mastectomies and Immediate-DElayed AutoLogous (IDEAL) breast reconstruction: the DIEP flap. Gland Surg 2016;5:24-31. [PubMed]
- Newbronner E, Atkin K. The changing health of Thalidomide survivors as they age: A scoping review. Disabil Health J 2018;11:184-91. [Crossref] [PubMed]
- Samel C, Albus C, Nippert I, et al. Life situation of women impaired by Thalidomide embryopathy in North Rhine-Westphalia - a comparative analysis of a recent cross-sectional study with earlier data. BMC Womens Health 2019;19:51. [Crossref] [PubMed]
- Peters KM, Albus C, Lüngen M, et al. Damage to Health, Psychosocial Disorders and Care Requirements of Thalidomide Survivors in North Rhine Westphalia from a Long-Term Perspective. Federal Health Centre North Rhine Westphalia, 2015.
- Ghassemi Jahani SA, Karlsson J, Brisby H, et al. Health-related quality of life and function in middle-aged individuals with thalidomide embryopathy. J Child Orthop 2016;10:691-703. [Crossref] [PubMed]
- Newbronner E, Glendinning C, Atkin K, et al. The health and quality of life of Thalidomide survivors as they age - Evidence from a UK survey. PLoS One 2019;14:e0210222. [Crossref] [PubMed]
- Pusic AL, Matros E, Fine N, et al. Patient-Reported Outcomes 1 Year After Immediate Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium Study. J Clin Oncol 2017;35:2499-506. [Crossref] [PubMed]
- Santosa KB, Qi J, Kim HM, et al. Long-term Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg 2018;153:891-9. [Crossref] [PubMed]
- Groß SE, Pfaff H, Swora M, et al. Health disparities among breast cancer patients with/without disabilities in Germany. Disabil Health J 2020;13:100873. [Crossref] [PubMed]
Cite this article as: Grünherz L, Fertsch S, Andree C, Munder B, Schulz T, Wolter A, Abdallah A. Autologous breast reconstruction in a patient with thalidomide embryopathy: case report. AME Surg J 2022;2:39.


