
Lung metastases from sarcoma: multidisciplinary approach—a narrative review
Introduction
Lung metastases are the principal cause of mortality in patients with primary bone (BS) and soft-tissue sarcoma (STS) and are accountable for worsening the prognosis in the presence of unresectable disease, giving a median survival of less than one year (1). Pulmonary metastases can be discovered in at least 20% of patients diagnosed with STS and 40% with a BS (2).
Indeed, the presence of metastatic disease at onset is very frequent. Besides, many studies, analyzing the effects of chemotherapy as first-line treatment of lung metastases from sarcoma, have reported limited or even no survival benefit (3). For patients with STS, the response rate to common chemotherapy is roughly reported at 25%, with a median OS of about 12 months (4).
Although pulmonary localizations are the expression of advanced disease, surgical resection has been proven as a fundamental treatment and a potentially curative option for those with resectable lung metastases, since when the International Registry of Lung Metastases (IRLM), in 1997, asserted that pulmonary metastasectomy (PM) is associated with improved outcomes (5). Improved survival has been further observed in several studies between patients who underwent metastasectomy compared with those who did not: the 5-year overall survival (OS) rate was reported of 17% among patients who underwent non-surgical treatment, whereas 3- and 5-year OS rates were 28–35% and 21–38%, respectively, following resection of lung metastases (6). In a more recent retrospective study, Shimizu and colleagues demonstrated a 3- and 5-year OS of 62 and 53%, with none of the patients in the non-surgical category who survived at 3 years (7).
There remains, however, the debate concerning which patients might gain most from surgery or what treatment has the best outcomes. Given the lack of strong literature evidence, and the various results reported for chemotherapy, radiotherapy, and surgical treatment, the management of patients with metastatic sarcoma should require a multidisciplinary approach with the collaboration of medical oncologists, radiotherapists, and thoracic surgeons, to build a tailored plan for each patient. We present the following article following the Narrative Review reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-68/rc).
Methods
We conducted a literature search on PubMed databases through May 31st, 2021, with the following keywords: PM, lung metastases, pulmonary sarcoma metastases, metastatic sarcoma treatment.
We retrieved articles concerning the management of lung metastasis from soft tissue and BS, aiming to clarify its current treatments options and indications.
The references of all analyzed articles were screened for relevant papers not found in the initial search.
Indications and results of chemotherapy and radiotherapy were obtained from most recent systematic reviews and meta-analysis. Studies with the best contributions for outcomes and prognostic factors of surgical PM, comprehending single and multiple-center experience and retrospective studies, were considered from an historical perspective to most modern evidence and reported in a narrative form.
Oncological background
Histology
Sarcomas are a variety of malignancies of mesenchymal origin consisting of nearly a hundred different histologic subtypes (8). They are commonly divided into the wide categories of soft-tissue and primary BS, with undifferentiated pleomorphic sarcoma and primary BS that are those who most easily metastasize to the lung (1,9).
Survival varies widely following the many different histologic subtypes. In a recent review, the 5-year OS rate for osteosarcoma is reported to be 34% compared to 25% for STS (10).
The most common histology among STS is the undifferentiated pleomorphic sarcoma (25%), followed by leiomyosarcoma (LMS) (18%), synovial sarcoma (10%), and liposarcoma (10%) (11). Neither of these subtypes represents an independent prognostic factor for survival per se, although, in some studies, patients with LMS metastases showed a favorable outcome compared to other histologies (12,13). LMS, specifically, seems to exhibit less aggressive tumor biology, which induces a significant OS advantage after PM (median survival 69.9 versus 23.9 months for other sarcoma subtypes); in addition, it tends to present with fewer pulmonary lesions and fewer lobes involvement than patients with non-LMS (14). Billingsley and colleagues also noted that patients with pulmonary metastases of malignant peripheral nerve sheath tumors and liposarcoma had a worse survival (1).
Disease-free interval (DFI)
The DFI, defined as the time between the treatment of primary tumor and the diagnosis of lung metastases, is directly associated with survival and a well-established prognostic factor. The IRLM agreed that a DFI of less than 36 months is correlated with poor survival (5). Furthermore, its efficacy in predicting survival after resection of lung metastases from sarcoma has been repeatedly confirmed with different cut-offs ranging from 11 to 34 months (11-13,15), probably reflecting different criteria of patient selection.
Besides, synchronous metastases, which are visible on initial staging imaging of the primary tumor and, by definition, have no DFI, are reported in a percentage around 32% of patients and should not be considered an absolute contraindication for PM (16). In a recent multivariate analysis, synchronous and metachronous metastases groups exhibit different baseline characteristics, but both showed comparable survival. Median DFI for patients with metachronous metastases is 27.7 months (16,17).
Number of metastatic foci
The number of metastatic nodules, accepted as a measure of tumor burden, is frequently used in patient selection for surgery. However, there is no general agreement about the maximum number of pulmonary metastases that limit surgical resection. Several surgical series have been shown the importance of number, size, or bilateral vs. unilateral disease as a prognostic indicator (12,18), but others did not demonstrate a significant difference in survival between patients with more or less than 4 lesions (11,19).
Nevertheless, effects on survival are uniformly reduced whenever complete resection is achieved (20).
Completeness of resection
Radical resection is considered the main goal of surgical treatment and has proved to be the most significant predictor of long-term survival following PM. In particular, incompleteness is a worse prognostic factor than DFI and multiple metastases combined, leading the median survival to 14 months instead of 24 months in the IRLM (5).
Interestingly, Billingsley et al. reported that patients with incompletely resected disease had only a marginal statistical survival difference compared with subjects treated with non-surgical treatments such as chemotherapy or radiotherapy alone (16.4 vs. 33.5 months for a complete resection) (1).
However, even when radical resection is achieved, in the majority of cases, the disease will eventually recur. A recent study at Rizzoli Orthopedic Hospital shows that 76.2% of patients had recurrent lung metastases after initial PM and that the risk is correlated with a DFI less than 1 year (21,22).
The role of repeated metastasectomy will be discussed below.
Medical treatments
Chemotherapy
There is no standard chemotherapy strategy for the treatment of metastatic sarcoma. The results of initial clinical trials were often “confounded” by grouping results from biologically different subtypes, which are characterized by different degrees of chemosensitivity (23).
In recent times, a histology-tailored approach has led to improved clinical outcomes in patients with more aggressive histology, such as Ewing sarcoma and osteosarcoma (24).
Currently, single-agent anthracycline-based therapy is considered the standard first-line therapy in advanced STS, associated with improved OS; a multi-agent combination of doxorubicin and ifosfamide is reserved for patients with adequately chemosensitive histology, and patients who are being considered for multimodality therapy and surgical resection. Promising results were also demonstrated with newer cytotoxic agents, including eribulin, trabectedin, and aldoxorubicin (25,26).
Standard chemotherapy for osteosarcoma metastatic at initial diagnosis is based on the MAP regimen (high-dose methotrexate, doxorubicin, and cisplatin, with the possible addition of ifosfamide or etoposide for recurrent cancer), raising the response rate of 15% for single-agent to nearly 40% (27,28). To date, the best 5-year survival rate reported in the literature for patients with osteosarcoma metastatic at presentation is 47% (29).
Therapeutic options involving the immune system are highly attractive and rapidly expanding. In a recent review, emerged that even the immune microenvironment is highly variable in STS, the strong immune presence in some subtypes offers a promise for immunotherapy, and several ongoing phase I/II trials are assessing the role of anti–PD-L1 agents (30).
However, as seen with conventional chemotherapy drugs, tumors utilize multiple pathways to resist immunotherapy, suggesting that combination approaches will still be needed to achieve meaningful and durable responses (31).
The effectiveness of conventional chemotherapy in addition to surgical metastasectomy is still controversial. Almost all surgical case series are influenced by a selection bias, that originates in the fact that chemotherapy is usually employed in patients with more aggressive diseases. At Memorial Sloan-Kettering Cancer Center two cohorts of patients with STS were compared, receiving PM alone or in combination with perioperative chemotherapy. The results of this study showed no difference in terms of OS in both groups (3).
However, despite the questionable benefit on survival, neoadjuvant chemotherapy seems to offer the convenience of better assessing tumor response to treatment before surgical resection, thus serving as a prognostic factor. Stephens and colleagues showed that patients without tumor progression while on chemotherapy had a median survival after metastasectomy of 35.5 months, whereas progression on chemotherapy is associated with worse outcomes (17.2 months) (32).
Radiation therapy
Radiotherapy for lung metastases has been substantially reserved for patients who are excluded from surgery. Except for Ewing sarcoma, which is exclusively radiosensitive (33), whole-lung irradiation (WLI) is generally not performed in metastatic disease (6).
Few studies described the experience of CT-guided radiofrequency ablation (RFA) of lung metastases, showing a 3-year survival rate not superior to 65.2% (34,35). More consistent results come from Stereotactic Body Radiation Therapy (SBRT); even many sarcoma subtypes have traditionally been considered radioresistant, the high-dose, hypofractionated SBRT gains a significant response in several studies, showing a 5-year OS ranging from 50.0% to 60.5% (36,37). Yu et al. reported an equivalent 4-year progression-free survival rate and post-relapse OS in the stereotactic radiation group compared to surgery for metastatic osteosarcoma (38).
Nevertheless, when lung metastases are completely resected, administering adjuvant radiotherapy still lacks undeniable benefits and is occasionally reserved in patients with positive margin resection or for residual gross disease (39).
Surgical management
Preconditions to metastasectomy, to undergo potentially curative surgery, are essentially associated with patient and disease status: the patient should tolerate lung resections; primary cancer must be either controlled or controllable with no evidence of active disease; complete resection must be achievable; the absence of extra-thoracic disease, however, this should not be an absolute contraindication if they are also suitable for resection or are already resected successfully; and, finally, there should be no better-proved treatment option to treat metastasis (40,41).
Planning a PM for metastatic sarcoma requires careful analysis of each case individually. The decision to proceed with surgery must consider the relative risks and benefits of all other therapeutic options and should be discussed in a multidisciplinary meeting between surgeons, oncologists, and radiotherapists.
Timing of surgery
The timing of surgery is one of the key points in the management of metastatic sarcoma disease. Indeed, there’s not a uniform consensus between surgeons who take an aggressive attitude and others who allow for a diagnostic interval between diagnosis and resection. The first approach is mainly based on technical considerations and the risk that growing nodules would determine more extensive resections or threaten completeness, the latter on the evidence that metastases behavior could yield a clue of disease course, especially under chemotherapy (6).
Available data indicate that both early or delayed surgery, do not have advantages. Except for patients with few lesions or long DFI, in which preemptive operations are more clearly indicated (42).
In general, the timing of metastasectomy should depend on patients’-tailored multidisciplinary discussion, and postponing surgery seems justified if the indication for resection is questionable due to a high risk of early recurrence.
Technique
Traditionally, bimanual palpation, during lung metastasectomy, has been considered the most appropriate approach to achieve complete resection of all metastatic lesions, including small nodules not identified on preoperative imaging (43).
Median sternotomy & clamshell thoracotomy
They have the advantage to allow contemporaneous access to both pleural cavities. Sternotomy, however, limits the exposure of the posterior hila and lower lobes. Such extensive surgical incisions, which were frequent in the early with the rationale of exploring both lungs, are nowadays, less and less used, in favor of more conservative approaches that have shown comparable oncological results (44).
Posterolateral or muscle-sparing lateral thoracotomy
A traditional unilateral thoracotomy provides excellent visualization and allows the surgeon to examine the entire parenchyma and pleural surface. Some authors initially expressed controversy about performing only unilateral thoracotomy, even in the presence of unilateral disease by imaging, giving that occult contralateral nodules could affect survival. Younes and colleagues, though, found that delaying contralateral thoracotomy until disease became radiologically apparent did not affect OS (45). The sequential thoracotomy, planned at least one month after the first operation, offers an acceptable option in the management of bilateral metastatic disease at onset and is nowadays quite popular (46).
Video-assisted thoracoscopic surgery (VATS)
VATS techniques have progressively gained a leading role in the management of many thoracic conditions. Some of the initial controversies consisted in its apparent limited ability to identify small lung lesions through minimally invasive incisions, thus the possible applications for PM (47). Eckardt and colleagues performed a prospective evaluation of thoracoscopic versus open metastasectomy, identifying by thoracoscopy only 87% of the nodules who were seen on preoperative CT scan. In contrast, with thoracotomy more additional nodules were discovered, 33% of which were metastatic lesions (48). However, should be noted that some authors including Cerfolio, identified by bimanual palpation nodules that were not detected by preoperative CT scan, but in little less than half of the patients, those missed lesions were malignant, resulting in the resection of about 20% more benign nodules with an open approach (49).
Similar supportive results were reported by Gossot and colleagues. The authors compared patients undergoing wedge resections by VATS and thoracotomy, for the treatment of sarcoma lung metastases. The analysis found similar overall survival and disease-free survival between the VATS and thoracotomy groups and underlined the well-known advantages of the minimally invasive approach, such as postoperative complications, pain, length of hospital stay, and providing patient’s quick surgical recovery and good quality of life, avoiding potential delay in adjuvant therapy (50).
Extent of resection
Negative margins and preservation of uninvolved pulmonary parenchyma is the goal of PM.
Erhunmwunsee and colleagues raised the argument that missing subclinical nodules result in inferior outcomes (43). Despite the lack of evidence that small indolent nodules have an impact on survival, surgeons should always strive for R0 resection because of the unpredictable biological behavior of the tumor.
If an R0 resection is achieved, performing an anatomic resection, such as segmentectomy or lobectomy, does not provide an additional benefit (51). However, for lesions that are technically challenging to remove by reason of location, segmentectomy may offer an advantage for achieving a complete resection while sparing lung parenchyma and maintaining adequate lung function (52).
In general, given the tendency of metastatic sarcoma to recur and, thus, the need for additional pulmonary resections, parenchyma-sparing approaches to preserve pulmonary function are critically important. Hence, non-anatomic wedge resections with stapler instruments are the most common intervention, especially in the presence of few nodules that can be safely approached by VATS (53).
When several foci must be targeted, multiple stapled wedge resections can lead to significant parenchymal distortion. Consequently, in our experience, the so-called “precision resection” is an alternative that is commonly considered. Nodules are removed opening the lung parenchyma through electrocautery and the residual defects are closed, after adequate hemostasis, by single or bidirectional locked suture of 3/0 of polypropylene with or without a combination of absorbable monofilament suture (MaxonTM) for the deeper layer.
With this technique, as is shown in Figure 1, dozens of small metastatic nodules could be resected preventing a drastic distortion of the parenchyma that will eventually compromise the entire lung function. A video of a precision resection is available online (Video 1).
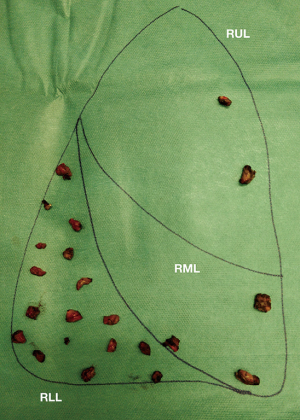
Extended resection & lymph node dissection
In selected patients, metastatic sarcoma may require lung resection with en bloc chest wall, pericardial, or diaphragm resection. These patients seem to have a significantly shorter median overall survival than those undergoing only lung nodules metastasectomy (54). However, when assessed to multivariate analysis, this difference loses significance, probably due to an association with incomplete resection. Therefore, extended surgery may be potentially curative when negative margins are achieved (39).
Extensive vascular resection and reconstruction, sleeve pulmonary resection, or pneumonectomy are feasible and justified in selected cases (long DFI, large tumor but eligible for complete excision) and were found to have admissible 30-day morbidity and mortality (38% vs. 0%, respectively) and a 5-year survival ranging from 19% to 52% (55,56). Tumors with mediastinal invasion into the heart and great vessels have also been reported to have favorable outcomes, unexpectedly superior to those accomplished with extended resections for primary lung cancer (57).
The need for routine lymphadenectomy during PM is still debatable, as the significance of nodal involvement is unclear and lymph node involvement is rare in sarcoma (58). Nodal metastases have been found in no more than 20% of patients with sarcoma intraoperatively, but some authors report that N1 disease is a prognostic indicator of impaired median survival (47.0 months for N0 and 18.3 months for N1) (59,60). To date, lymphadenectomy is not routinely recommended during resection of pulmonary metastases from sarcoma (61).
Recurrence and repeated metastasectomy
The pulmonary recurrence rate after a successful metastasectomy ranges between 30.6% to 69% for any malignancies, regardless of the surgical approach (44). Sarcomas, in particular, have a peculiar intrathoracic pattern of relapse accounting for 66% of all recurrences, remarkably different from that of other tumors that recur mainly distant, and the proportion of relapsing patients who undergo a second PM is significantly higher in sarcomas than in any other tumor type (53% vs. 28%) (5).
Repeated PMs are justified based on the evidence that improved long-term survival has been obtained in patients with STS, with the mandatory condition that a complete resection must be achieved. The 5-year overall survival following repeated resection is typically between 36% and 57% (20,62,63). Jaklitsch and colleagues also found that there is no limit to the number of metastasectomies a patient can undergo, indeed the 5-year OS remained greater than 33% for up to 4 procedures but tends to lower with 5 or more (64).
Conclusions
Pulmonary metastases are signs of advanced disease and always represent a challenge in the management of solid cancers. STS and BS exhibit a strong tendency to produce lung lesions that impair overall survival. Moreover, lung metastases could easily recur over time showing an inadequate response to systemic treatments.
PM is now widely accepted as the most effective therapy, even in the presence of multiple, bilateral lesions, and in relapse. Indeed, it is accepted despite the lack of prospective randomized controlled trials proving its efficacy and is based on many retrospective studies that showed a survival benefit for patients undergoing PM compared to patients undergoing non-surgical therapy. Due to the retrospective nature of these studies, they all suffer from inherent selection bias, but lots of surgical case series pointed out that the main reasons not to undergo surgery are usually related to a more extended disease or severe functional limitations and comorbidities.
This evidence is strengthened by the fact that, nowadays, medical treatments such as chemo- and radiotherapy, still lack effectiveness in the control of metastatic disease when used alone.
Advances have been made in many fields: novel chemotherapy agents and immunotherapy offers a promise in the control of systemic dissemination; Stereotactic Body Radiation Therapy gains a significant response in several studies; improvements in preoperative imaging have led to better identification and surveillance of lung metastases; and, finally, the spread of minimally invasive thoracic surgery techniques have offered the chance of most tolerable operations for the patients in terms of recovery and quality of life.
This narrative review had the goal of summarizing most of the current knowledge in the management of metastatic sarcomas, also by showing the inconsistency between different studies on the same aspect, such as medical treatment protocols, surgery indications or management of recurrences. To clarify this topic, avoiding selection bias and aiming to clinical relevance, larger randomized prospective, and possibly multicentric, studies are advocated.
Important results have been obtained through the years and more are expected in the future, but we believe that the treatment of this complex disease should always require a multidisciplinary approach involving oncologists, radiotherapists, and surgeons that could offer a tailored therapy for every patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Davide Tosi, Alessandro Palleschi and Paolo Mendogni) for the series “Management and Treatment of Lung Metastases” published in AME Surgical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-68/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-68/coif). The series “Management and Treatment of Lung Metastases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602-10; discussion 610-2. [Crossref] [PubMed]
- Cariboni U, De Sanctis R, Giaretta M, et al. Survival Outcome and Prognostic Factors After Pulmonary Metastasectomy in Sarcoma Patients: A 18-Year Experience at a Single High-volume Referral Center. Am J Clin Oncol 2019;42:6-11. [Crossref] [PubMed]
- Canter RJ, Qin LX, Downey RJ, et al. Perioperative chemotherapy in patients undergoing pulmonary resection for metastatic soft-tissue sarcoma of the extremity: a retrospective analysis. Cancer 2007;110:2050-60. [Crossref] [PubMed]
- Smith R, Demmy TL. Pulmonary metastasectomy for soft tissue sarcoma. Surg Oncol Clin N Am 2012;21:269-86. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Digesu CS, Wiesel O, Vaporciyan AA, et al. Management of Sarcoma Metastases to the Lung. Surg Oncol Clin N Am 2016;25:721-33. [Crossref] [PubMed]
- Shimizu J, Emori M, Murahashi Y, et al. Pulmonary metastasectomy is associated with prolonged survival among patients with bone and soft tissue sarcoma. Mol Clin Oncol 2020;12:429-34. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of Soft Tissue and Bone, 5th ed. Lyon, France: IARC Press, 2020.
- García Franco CE, Torre W, Tamura A, et al. Long-term results after resection for bone sarcoma pulmonary metastases. Eur J Cardiothorac Surg 2010;37:1205-8. [Crossref] [PubMed]
- Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736. [Crossref] [PubMed]
- Dossett LA, Toloza EM, Fontaine J, et al. Outcomes and clinical predictors of improved survival in a patients undergoing pulmonary metastasectomy for sarcoma. J Surg Oncol 2015;112:103-6. [Crossref] [PubMed]
- Blackmon SH, Shah N, Roth JA, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg 2009;88:877-84; discussion 884-5. [Crossref] [PubMed]
- Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg 2015;149:85-92. [Crossref] [PubMed]
- Burt BM, Ocejo S, Mery CM, et al. Repeated and aggressive pulmonary resections for leiomyosarcoma metastases extends survival. Ann Thorac Surg 2011;92:1202-7. [Crossref] [PubMed]
- van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 1996;77:675-82. [Crossref] [PubMed]
- Stork T, Boemans R, Hardes J, et al. Number of metastases and their response to chemotherapy impact survival of patients with isolated lung metastases from bone-derived sarcoma. BMC Cancer 2021;21:375. [Crossref] [PubMed]
- Outani H, Hamada K, Yasuda N, et al. Impact of surgical resection on survival in patients with metastatic soft tissue sarcoma and comparison between synchronous and metachronous metastases. J Orthop Sci 2022;27:892-8. [Crossref] [PubMed]
- Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcoma: prognostic and surgical implications from histology. Interact Cardiovasc Thorac Surg 2012;15:612-7. [Crossref] [PubMed]
- Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the "international registry of lung metastases". J Thorac Oncol 2011;6:1373-8. [Crossref] [PubMed]
- Rehders A, Hosch SB, Scheunemann P, et al. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg 2007;142:70-5; discission 76.
- Tetta C, Rocca M, Salone M, et al. Predictors of lung recurrence and disease-specific mortality after pulmonary metastasectomy for soft tissue sarcoma. Surg Oncol 2021;37:101532. [Crossref] [PubMed]
- Tanju S, Saricam M, Kaba E, et al. Factors associated with pulmonary recurrence after pulmonary metastasectomy for sarcomatous disease. Surg Today 2014;44:914-8. [Crossref] [PubMed]
- Linch M, Miah AB, Thway K, et al. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol 2014;11:187-202. [Crossref] [PubMed]
- Dancsok AR, Asleh-Aburaya K, Nielsen TO. Advances in sarcoma diagnostics and treatment. Oncotarget 2017;8:7068-93. [Crossref] [PubMed]
- Liebner DA. The indications and efficacy of conventional chemotherapy in primary and recurrent sarcoma. J Surg Oncol 2015;111:622-31. [Crossref] [PubMed]
- Zer A, Prince RM, Amir E, et al. Multi-agent chemotherapy in advanced soft tissue sarcoma (STS) - A systematic review and meta-analysis. Cancer Treat Rev 2018;63:71-8. [Crossref] [PubMed]
- Tsukamoto S, Errani C, Angelini A, et al. Current Treatment Considerations for Osteosarcoma Metastatic at Presentation. Orthopedics 2020;43:e345-58. [Crossref] [PubMed]
- Meazza C, Asaftei SD. State-of-the-art, approved therapeutics for the pharmacological management of osteosarcoma. Expert Opin Pharmacother 2021;22:1995-2006. [Crossref] [PubMed]
- Chou AJ, Kleinerman ES, Krailo MD, et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer 2009;115:5339-48. [Crossref] [PubMed]
- Ayodele O, Razak ARA. Immunotherapy in soft-tissue sarcoma. Curr Oncol 2020;27:17-23. [Crossref] [PubMed]
- Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr Blood Cancer 2018;65:e27227. [Crossref] [PubMed]
- Stephens EH, Blackmon SH, Correa AM, et al. Progression after chemotherapy is a novel predictor of poor outcomes after pulmonary metastasectomy in sarcoma patients. J Am Coll Surg 2011;212:821-6. [Crossref] [PubMed]
- Casey DL, Alektiar KM, Gerber NK, et al. Whole lung irradiation for adults with pulmonary metastases from Ewing sarcoma. Int J Radiat Oncol Biol Phys 2014;89:1069-75. [Crossref] [PubMed]
- Palussière J, Italiano A, Descat E, et al. Sarcoma lung metastases treated with percutaneous radiofrequency ablation: results from 29 patients. Ann Surg Oncol 2011;18:3771-7. [Crossref] [PubMed]
- Koelblinger C, Strauss S, Gillams A. Outcome after radiofrequency ablation of sarcoma lung metastases. Cardiovasc Intervent Radiol 2014;37:147-53. [Crossref] [PubMed]
- Lindsay AD, Haupt EE, Chan CM, et al. Treatment of Sarcoma Lung Metastases with Stereotactic Body Radiotherapy. Sarcoma 2018;2018:9132359. [Crossref] [PubMed]
- Navarria P, Ascolese AM, Cozzi L, et al. Stereotactic body radiation therapy for lung metastases from soft tissue sarcoma. Eur J Cancer 2015;51:668-74. [Crossref] [PubMed]
- Yu W, Liu Z, Tang L, et al. Efficacy and safety of stereotactic radiosurgery for pulmonary metastases from osteosarcoma: Experience in 73 patients. Sci Rep 2017;7:17480. [Crossref] [PubMed]
- Smith R, Pak Y, Kraybill W, et al. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol 2009;35:356-61. [Crossref] [PubMed]
- Pastorino U. Lung metastasectomy: why, when, how. Crit Rev Oncol Hematol 1997;26:137-45. [Crossref] [PubMed]
- Sudarshan M, Murthy SC. Current Indications for Pulmonary Metastasectomy. Surg Oncol Clin N Am 2020;29:673-83. [Crossref] [PubMed]
- Krüger M, Schmitto JD, Wiegmann B, et al. Optimal timing of pulmonary metastasectomy--is a delayed operation beneficial or counterproductive? Eur J Surg Oncol 2014;40:1049-55. [Crossref] [PubMed]
- Erhunmwunsee L, D'Amico TA. Surgical management of pulmonary metastases. Ann Thorac Surg 2009;88:2052-60. [Crossref] [PubMed]
- Cheung FP, Alam NZ, Wright GM. The Past, Present and Future of Pulmonary Metastasectomy: A Review Article. Ann Thorac Cardiovasc Surg 2019;25:129-41. [Crossref] [PubMed]
- Younes RN, Gross JL, Deheinzelin D. Surgical resection of unilateral lung metastases: is bilateral thoracotomy necessary? World J Surg 2002;26:1112-6. [Crossref] [PubMed]
- Ripley RT, Downey RJ. Pulmonary metastasectomy. J Surg Oncol 2014;109:42-6. [Crossref] [PubMed]
- Downey RJ, Bains MS. Open Surgical Approaches for Pulmonary Metastasectomy. Thorac Surg Clin 2016;26:13-8. [Crossref] [PubMed]
- Eckardt J, Licht PB. Thoracoscopic or open surgery for pulmonary metastasectomy: an observer blinded study. Ann Thorac Surg 2014;98:466-70. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, McCarty TP, et al. A prospective study to determine the incidence of non-imaged malignant pulmonary nodules in patients who undergo metastasectomy by thoracotomy with lung palpation. Ann Thorac Surg 2011;91:1696-700; discussion 1700-1. [Crossref] [PubMed]
- Gossot D, Radu C, Girard P, et al. Resection of pulmonary metastases from sarcoma: can some patients benefit from a less invasive approach? Ann Thorac Surg 2009;87:238-43. [Crossref] [PubMed]
- Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg 2014;3:176-82. [PubMed]
- Leshnower BG, Miller DL, Fernandez FG, et al. Video-assisted thoracoscopic surgery segmentectomy: a safe and effective procedure. Ann Thorac Surg 2010;89:1571-6. [Crossref] [PubMed]
- Perentes JY, Krueger T, Lovis A, et al. Thoracoscopic resection of pulmonary metastasis: current practice and results. Crit Rev Oncol Hematol 2015;95:105-13. [Crossref] [PubMed]
- Ueda T, Uchida A, Kodama K, et al. Aggressive pulmonary metastasectomy for soft tissue sarcomas. Cancer 1993;72:1919-25. [Crossref] [PubMed]
- Casiraghi M, Maisonneuve P, Brambilla D, et al. The role of extended pulmonary metastasectomy. J Thorac Oncol 2015;10:924-9. [Crossref] [PubMed]
- Migliore M, Jakovic R, Hensens A, et al. Extending surgery for pulmonary metastasectomy: what are the limits? J Thorac Oncol 2010;5:S155-60. [Crossref] [PubMed]
- Wiebe K, Baraki H, Macchiarini P, et al. Extended pulmonary resections of advanced thoracic malignancies with support of cardiopulmonary bypass. Eur J Cardiothorac Surg 2006;29:571-7; discussion 577-8. [Crossref] [PubMed]
- Fong Y, Coit DG, Woodruff JM, et al. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 1993;217:72-7. [Crossref] [PubMed]
- Pfannschmidt J, Klode J, Muley T, et al. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg 2006;81:448-54. [Crossref] [PubMed]
- Veronesi G, Petrella F, Leo F, et al. Prognostic role of lymph node involvement in lung metastasectomy. J Thorac Cardiovasc Surg 2007;133:967-72. [Crossref] [PubMed]
- Kon Z, Martin L. Resection for thoracic metastases from sarcoma. Oncology (Williston Park) 2011;25:1198-204. [PubMed]
- Liebl LS, Elson F, Quaas A, et al. Value of repeat resection for survival in pulmonary metastases from soft tissue sarcoma. Anticancer Res 2007;27:2897-902. [PubMed]
- Kandioler D, Krömer E, Tüchler H, et al. Long-term results after repeated surgical removal of pulmonary metastases. Ann Thorac Surg 1998;65:909-12. [Crossref] [PubMed]
- Jaklitsch MT, Mery CM, Lukanich JM, et al. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg 2001;121:657-67. [Crossref] [PubMed]
Cite this article as: Gherzi L, Ferrari M, Pardolesi A. Lung metastases from sarcoma: multidisciplinary approach—a narrative review. AME Surg J 2022;2:38.


