
Do we even need to relax? Evolution of non-intubated video assisted thoracic surgery thymectomy for myasthenia gravis—a narrative review
Introduction
Thymus and myasthenia gravis (MG)
With the recent exuberant development of day to day life, and mankind leaping with giant steps toward making life easier and more accessible, turns to recyclable energy and resources and casts its eyes toward distant planets to colonize, thoracic surgery introduces video-assisted thoracic surgery (VATS) as the minimally invasive approach and moves further to the use of only a single port, granting significantly decreased overall burden to the patient, and at the same time creating new technical challenges for surgeons.
Ever since its first description in the 1800s, the gland positioned next to the heart earned a special and somewhat mystical role as the seat of the soul, an attribute given to the thymus by the Greeks (1). After the first partial thymectomy in 1896 by Ludwig Rehn and with Sauerbruch performing the first transcervical total thymectomy in 1911, management of thymic disorders gradually infiltrated the general and thoracic surgery practice.
Thymic lesions account for a high percentage of mediastinal masses (20–25% of all mediastinal tumors and 50% of the anterior mediastinum) (2). The first connection between a mediastinal mass and MG was established by Weigert in 1901 (3).
MG is an autoimmune disorder with main features of muscle weakness and fatigue. Although only 15% of thymoma patients present with MG, thymomas account for 15% of mediastinal masses (4). Thymic masses have been chategorized by the World Health Organization (WHO) in 2004 redefined by International Thymic Malignancy Interest Group (ITMIG) in 2014 and fundamental staging proposed by Masaoka in 1981 (5,6). The Osserman system has been used for clinical classification of symptoms from involvement limited to the ocular region to acute severe myasthenia (7).
Thymectomy for MG became an established treatment option since Blalock et al. reported significant improvement of MG-linked symptoms after thymectomy in 1939 (8). Since then there have been numerous studies confirming this connection (4,9,10). We present the following article in accordance with the Narrative Review reporting checklist (available at https://asj.amegroups.com/article/view/10.21037/asj-21-61/rc).
Objective
Improvements in surgical attitude, innovation and armamentarium have evolved steadily from traditional thymectomy via sternotomy to traditional-, and uniportal VATS to the most recent non-intubated VATS (NI-VATS) thymectomy. The current review describes evolution of the NI minimally invasive technique and attempts to present benefits and backdrops of NI-VATS thymectomy from an objective aspect.
Methods
Literature search was conducted including years: 1981–2021, through PubMed, PubMed Central and Scopus databases. Only already published manuscripts written in English language were considered including case reports, retrospective-, and prospective cohorts, meta-analysis, systematic reviews and randomized controlled trials.
Discussion
NI-VATS
Double-lumen intubation is a standard approach during thoracic surgery procedures. However with VATS gradually replacing traditional sternotomy, the NI spontaneous ventilation technique has also been proposed, first by Pompeo et al. in 2004, and since then many more, describing advantages of the new technique (11). In recent years NI-VATS has been thoroughly investigated both from the surgical-, and anaesthesiological aspect, and has been granted the benefit of reducing negative effects of traditional intubation and general anaesthesia (12). Since no muscle relaxation is needed during surgery, it has also shown promising results in faster recovery of normal muscle tone (13). According to the meta-analysis of Wen et al., including 27 studies and 2,537 cases (NI-VATS n=1,283, I-VATS n=1,254), significantly improved results were seen favoring NI-VATS in terms of postoperative fasting time, hospital stay, operative- and anaesthesia time and mortality rate (14). NI-VATS has successfully been applied during various thoracic procedures including pneumothorax (PTX)/pleural effusions/empyaema, lung volume reduction, or thymectomy, carried out by traditional-, or uniportal VATS (15-17). Furthermore, the feasibility and safety of NI-RATS (robot assisted thoracic surgery) carinal resection has recently been reported in a preliminary study by Li et al. in selected cases body mass index (BMI) <30] combining advantages of the minimally invasive approach with 3D vision and improved access during intrathoracic suturing (18).
Anaesthesia considerations—preoperative evaluation and preparation
Preoperative evaluation of MG patients should be based on disease severity according to Osserman’s classification and treatment regimen. Extra attention should be paid to voluntary and respiratory muscles. Respiratory muscle strength can be measured by pulmonary function tests (negative inspiratory pressure and forced vital capacity) (19).
Preoperative management of the myasthenic patient is influenced by the surgical procedure, surgeon preference and the anaesthesiologist, who can choose to omit anticholinesterase inhibitor (AChI) medication on the morning of surgery to decrease the need of muscle relaxants (20). Substitution of AChI with intravenous alternatives can also be carried out. Steroid-dependent patients should be monitored during the perioperative period. Anxiolytic, sedative-, and opioid premedications are rarely given to patients, however in case of mainly ocular symptoms, a small dose of sedatives may be acceptable.
Response to neuromuscular blockers
Neuromuscular blocking agents act by interrupting neuromuscular transmission at the level of nicotinic acetylcholine receptors at the motor end plate. This mode of action can be classified as antagonist (nondepolarizing) or agonist (depolarizing) (21). The myasthenic patient is typically sensitive to nondepolarizing neuromuscular blockers. Long-acting muscle relaxants such as, pancuronium and pipecuronium—which are intermediate—and short-acting nondepolarizing agents—can be administered with continuous monitoring of neuromuscular transmission (22). Patients with MG show resistance to depolarizing neuromuscular blockers (succinylcholine). It is presumed that requirements are increased due to the loss of receptors, since these agents create neuromuscular blockade through agonist action.
Intravenous anaesthetic agents
Anaesthetic management of MG patients through total intravenous technique using propofol has been described without deleterious effects (23). Propofol has the theoretic advantage of short duration of action without affecting neuromuscular transmission. Opioid analgesics given in therapeutic concentrations do not appear to depress neuromuscular transmission in myasthenic muscle tissue. However, opioids with normal dose might cause central respiratory depression. The introduction of short-acting opioids makes these drugs more simple to titrate. Remifentanil, with its short elimination half-life (9.5 minutes) makes this drug an appealing alternative (24).
Regional anaesthesia
Potentiation of drugs acting as neuromuscular blockers through local anaesthetics has been previously reported. These agents decrease sensitivity of the postjunctional membrane to acetylcholine. Ester-based anaesthetics, metabolized by cholinesterase, may present particular problems in patients taking anticholinesterase medications. Regional- and local anaesthesia should be carried out using reduced doses of amide (rather than ester) as local anaesthetics in order to avoid increased blood levels (23).
Anaesthesia for NI-VATS and I-VATS
Analgesia during NI-VATS thymectomy mainly involves thoracic epidural anaesthesia (TEA), paravertebral blockade (PVB) and/or intercostal nerve blockade (INB). Excluding the effects of muscle relaxants reduces the incidence of MC and warrants faster recovery after surgery (25). However epidural anaesthesia is not risk-free, and may lead to hypotension, urinary retention or bradycardia, although the overall complication rate after TEA was reported by Gieble et al. to be 3.1% (26-28). PVB by avoiding contralateral sympathectomy may manage to evade adverse effects such as hypotension and may provide postoperative (after thoracotomy or VATS) pain relief comparable with TEA (29). Intercostal nerve-blockade with bupivacain 0.5% can be performed percutaneously or more often from the thoracic cavity. It may offer less efficient pain control when compared to TEA or PVB, however accompanying adverse effects are also significantly reduced (30).
Anaesthesia during VATS should not only maintain sufficient depth, but also control lung expansion and intrathoracic pressure (31). Double-lumen intubation and one-lung ventilation (OLV) has become essential for thoracic and mediastinal procedures. On the other hand with the goal of excluding intubation-associated complications—such as pulmonary injuries due to positive pressure, respiratory infections, arrhythmia—NI ventilation has become common practice for minor and later major thoracic procedures (17,18). The formation of an artificial PTX during VATS is essential for lung collapse in order to maintain operating field with sufficient visualization. During NI-VATS the patient remains sedated without the use of muscle relaxants, thus chest movements and involuntary cough-reflex remain major issues to deal with. INB with lidocaine/bupivacaine can significantly reduce cough-reflex, and facilitate intrathoracic maneuvering, making NI-VATS a safe and reliable method for various thoracic procedures. Without the use of an intratracheal tube and the exclusion of muscle relaxants, complications of intubated surgery—bronchospasm, cardiac disorders, post-extubation throat irritation—can be reduced, warranting faster patient recovery (ERAS; enhanced recovery after surgery) (32). Without the use of muscle relaxants, prolonged muscle recovery is eliminated with ventilatory capacity (VC) rapidly returning to normal range after surgery. Nevertheless patient recovery strongly depends on postoperative inflammatory response. Alveolar inflammatory response during OLV is associated with the increased release of acute inflammatory cytokines (due to lung trauma-ischaemia-reperfusion during OLV), which has a high impact on postoperative complications (33). A 2021 RCT by Jeon et al. compared inflammatory response during intubated-, and NI anaesthesia after major lung resections (lobectomy), and confirmed decreased levels of IL-6 and TNF-α in cases of NI cases (34). A meta-analysis by Zhang et al. based on 14 RCTs, also reported technical outcomes, such as surgical field satisfaction (SFS), and anaesthesia satisfaction scores (ASC). SFS was measured on a scale from 1–4 (1: best vision and lung collapse; and 4: worst exposure to the surgical field and the need to convert to intubation), and did not confirm significant difference in NI-VATS vs. I-VATS, however ASC proved to be significantly higher in cases of NI-VATS (35). With the spread of NI-VATS, the importance of careful patient selection also needs to be addressed. Major contraindications for NI-VATS include hemodinamic instability, anticipated difficult airway, BMI >30 and central pulmonary lesion which should be kept a priority (36). An increased risk of aspiration can occur in cases of hiatal hernia, or untreated gastroesophageal reflux disease. Caution should be taken in patients with impaired pulmonary function and chronic obstructive pulmonary disease (COPD), due to a higher risk of postoperative respiratory failure. However, hypoxemia during NI-VATS is in most cases transient and can readily be improved by high-flow humidified oxygen (37). Careful patient selection, strict postoperative care and regular follow-up are all essential in order to decrease overall complication rates, with potential conversion to intubated general anesthesia without delay.
Results have also shown that overall complication rate was lower in NI-VATS [odds ratio (OR) 0.41; 95% CI: 0.25 to 0.67; P=0.0004], with decreased incidence of air-leaks (OR 0.45; 95% CI: 0.24 to 0.87; P=0.02) and postoperative hoarseness (OR 0.06; 95% CI: 0.02 to 0.21; P<0.00001) (35).
Long term results comparing the two methods may also add a surplus to the dilemma between intubated-, and NI-VATS (38). NI-VATS from a subxiphoid approach has also been performed for the resection of anterior mediastinal masses showing comparable results with I-VATS in terms of intra-, and postoperative complications, thus the method may serve as an alternative in predicted cases of difficult airways (39). In order to reduce negative effects of OLV, other methods in addition to purely spontaneous ventilation have also been described, such as spontaneous ventilation combined with intubation (SVI). During SVI after vagus nerve blockade with bupivacaine, the double-lumen tube is introduced, maintaining spontaneous ventilation. Furák et al. reported less strict exclusion criteria for SVI than for non-intubated thoracic surgery (NITS) and a 77% reduction in OLV, successfully performing VATS- and open lobectomies with SVI (40). Beneficial effects of the NI approach have undoubtedly made the method worthy of worldwide appliance, however some limitations should also be mentioned. Excessive mediastinal movements during NI surgery are a major factor influencing technical feasibility of the procedure, hence these operations are usually not recommended in patients with extreme body mass index (BMI). Intrathoracic adhesions may also significantly limit working space and can further complicate, or potentially hamper NI-VATS (41), a probable reason for many surgeons not to acquire the NI technique.
Spontaneous ventilation thymectomy for MG
Thymectomy for MG has been the mainstay of treatment and with the introduction of VATS thymectomies, perioperative morbidity, hospital stay and operative costs have dropped significantly as opposed to open thymectomy (42). Pushing boundaries further—as in cases of VATS for major pulmonary resections—the NI approach has also been introduced for thymectomies, initially with the traditional multiportal-, and later the uniportal approach. The main goal of the NI technique is clearly to reduce adverse effects of traditional double-lumen intubation (lung infections, postoperative dysphagia-, and hoarseness, trauma of the laryngo-pharynx, etc.) with keeping patient safety first. In a study by Liu et al., in which 225 patients underwent either uniportal NI-VATS (n=96), or uniportal I-VATS thymectomy (n=129) for mediastinal masses, results suggested that there was no significant difference among the two groups in terms of mean operative time (61 vs. 55 min), or lowest SpO2 values (uNI-VATS vs. uI-VATS: 96.1 vs. 98.6; P=0.38) (43).
In addition to well-known advantages of the NI approach, MG patients may readily benefit from the exclusion of volatile anaesthetic agents and muscle relaxants which may potentially induce myasthenic crisis (MC). In addition, the estimated incidence of post-thymectomy MC is reported to be between 6–34% (44). According to the study by Jiang et al. comparing 36 NI-VATS thymectomies (using a thoracic epidural catheter and laryngeal mask for oxygen flow) with 68 intubated VATS thymectomies, MC occurred in 20.6% and 2.8% during I-VATS and NI-VATS, respectively with reduced postoperative VAS score and shorter length of hospital stay also favoring the NI-VATS thymectomy group (25). Besides numerous negative effects, the length of intubation also plays a pivotal role in the development of complications, hence making operative time a crucial factor. Jiang et al. reported a 120-minute operative time for a 3D subxiphoid uniportal VATS thymectomy and mean duration of surgery to be 134 minutes for 27 NI-VATS thymectomies performed by the same surgical team (compared with I-VATS thymectomy: 159.9 min) (25,45). Operative time of VATS vs. open thymectomy has previously been compared in the 2015 systematic review by Xie et al. which showed no significant difference between the two groups [VATS thymectomy vs. open thymectomy: 172 min (117.0–249.8) vs. 173.6 (131.0–227.9)] and which are comparable (and even shorter) with results of NI-VATS thymectomy (134 minutes) (46).
Surgical considerations
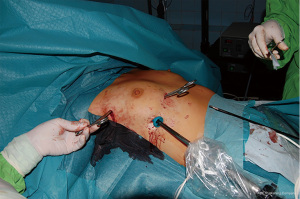
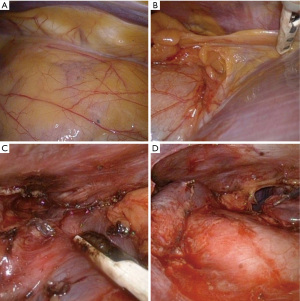
The optimal surgical approach to VATS thymectomy remains a question of debate as there are numerous options described for minimal access surgery. VATS thymectomy may be carried out from eighter side of the chest wall, using the subxiphoid- or transcervical approach and with the combination of these methods (47) (Figure 1). Approach mainly depends on surgeon preference and laterality of the treated condition. Positioning of the patient during surgery may be carried out in a lithotomy or semi-supine position for optimal exposure (43,25). Al-Abdullatief et al. reported a series of 25 patients operated from the left side, while Matsumoto et al. published awake surgery from a subxiphoid approach (13,48). Pompeo et al. reported a 3 patient-series with MG (2 Masaoka stage I and 1 Masaoka stage IIa) performing single-incision spontaneous ventilation VATS thymectomy from the right side, with short hospital stay, no postoperative myasthenic crisis and R0 resections (49). Regardless of the operative approach, principles of thymectomy should always be kept in sight. Thymectomy besides resection of the thymus and perithymic fat, should in each case involve removal of ectopic thymic tissue (ETT) since incidence of ETT (most frequently found in: perithymic fat, cardio-phrenic space, pretracheal fat, aorto-caval groove) has been reported to be 21–63%, and successful resection strongly correlates with improvement of MG symptoms (50). In current clinical practice preoperative imaging of ETT is not routinely carried out, a number of MRI and PET-CT reports state that in numerous cases ETT can be detected on the neck or in the mediastinum (51). In terms of MG thymectomies, it would be extremely advantageous if some information could be routinely obtained on imaging about the localization of ETT. Principles of surgical treatment of MG include complete removal of the thymus with perithymic fat and possible ETT (Figure 2). In MG cases usually two questions arise in terms of surgical treatment. (I) Does the patient have thymoma? (II) Is there any amount of ETT or “abnormal fat” around the thymus or in the mediastinum? In case ectopic foci remain in the mediastinum or on the neck, the postoperative improvement of MG is significantly worse.
Summary
With the exclusion of volatile anaesthetic agents and especially muscle relaxants the NI method presents promising results in terms of surgical feasibility and faster overall patient recovery. The reduction of intubation associated adverse effects holds a major advantage over traditional double-lumen intubation, however, further results based on higher patient numbers are needed to pinpoint the exact role and confirm outcomes of NI-VATS thymectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (József Furák) for the series “Spontaneous Ventilation Thoracic Surgery” published in AME Surgical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://asj.amegroups.com/article/view/10.21037/asj-21-61/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://asj.amegroups.com/article/view/10.21037/asj-21-61/coif). The series “Spontaneous Ventilation Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lavini C. The Thymus from Antiquity to the Present Day: the History of a Mysterious Gland. In: Lavini C, Moran CA, Morandi U, et al. editors. Thymus Gland Pathology. Milano: Springer, 2008.
- Kumar A, Regmi SK, Dutta R, et al. Characterization of thymic masses using (18)F-FDG PET-CT. Ann Nucl Med 2009;23:569-77. [Crossref] [PubMed]
- Müller-Hermelink HK, Marx A. Pathological aspects of malignant and benign thymic disorders. Ann Med 1999;31:5-14. [PubMed]
- López-Cano M, Ponseti-Bosch JM, Espin-Basany E, et al. Clinical and pathologic predictors of outcome in thymoma-associated myasthenia gravis. Ann Thorac Surg 2003;76:1643-9; discussion 1649. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Osserman KE. Myasthenia gravis. Grune-Stratton, New York: 1958:79-80.
- Blalock A, Mason MF, Morgan HJ, et al. Myasthenia gravis and tumors of the thymic region: report of a case in which the tumor was removed. Ann Surg 1939;110:544-61. [Crossref] [PubMed]
- Monden Y, Nakahara K, Kagotani K, et al. Myasthenia gravis with thymoma: analysis of and postoperative prognosis for 65 patients with thymomatous myasthenia gravis. Ann Thorac Surg 1984;38:46-52. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Jiang L, Liu J, Gonzalez-Rivas D, et al. Thoracoscopic surgery for tracheal and carinal resection and reconstruction under spontaneous ventilation. J Thorac Cardiovasc Surg 2018;155:2746-54. [Crossref] [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Wen Y, Liang H, Qiu G, et al. Non-intubated spontaneous ventilation in video-assisted thoracoscopic surgery: a meta-analysis. Eur J Cardiothorac Surg 2020;57:428-37. [PubMed]
- Furák J, Szabó Z, Horváth T, et al. Nem intubált, spontán légző betegnél, egy metszésből, minimálisan invazív módon elvégzett tüdőlebeny-eltávolítás mint új műtéti eljárás klinikánk gyakorlatában [Non-intubated, uniportal, video assisted thoracic surgery [VATS] lobectomy, as a new procedure in our department]. Magy Seb 2017;70:113-17. [PubMed]
- Rocco G. Non-intubated uniportal lung surgery†. Eur J Cardiothorac Surg 2016;49:i3-5. [Crossref] [PubMed]
- AlGhamdi ZM, Lynhiavu L, Moon YK, et al. Comparison of non-intubated versus intubated video-assisted thoracoscopic lobectomy for lung cancer. J Thorac Dis 2018;10:4236-43. [Crossref] [PubMed]
- Li S, Ai Q, Liang H, et al. Non-intubated Robotic-Assisted Thoracic Surgery for Tracheal/Airway Resection and Reconstruction: Technique Description and Preliminary Results. Ann Surg 2022;275:e534-6. [Crossref] [PubMed]
- Naguib M, el Dawlatly AA, Ashour M, et al. Multivariate determinants of the need for postoperative ventilation in myasthenia gravis. Can J Anaesth 1996;43:1006-13. [Crossref] [PubMed]
- Ha JC, Richman DP. Myasthenia gravis and related disorders: Pathology and molecular pathogenesis. Biochim Biophys Acta 2015;1852:651-7. [Crossref] [PubMed]
- Book WJ, Abel M, Eisenkraft JB. Adverse effects of depolarising neuromuscular blocking agents. Incidence, prevention and management. Drug Saf 1994;10:331-49. [Crossref] [PubMed]
- Khuenl-Brady KS, Wattwil M, Vanacker BF, et al. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: a multicenter, randomized, controlled trial. Anesth Analg 2010;110:64-73. [Crossref] [PubMed]
- Akpolat N, Tilgen H, Gürsoy F, et al. Thoracic epidural anaesthesia and analgesia with bupivacaine for transsternal thymectomy for myasthenia gravis. Eur J Anaesthesiol 1997;14:220-3. [Crossref] [PubMed]
- Ng JM. Total intravenous anesthesia with propofol and remifentanil for video-assisted thoracoscopic thymectomy in patients with myasthenia gravis. Anesth Analg 2006;103:256-7. [Crossref] [PubMed]
- Jiang L, Depypere L, Rocco G, et al. Spontaneous ventilation thoracoscopic thymectomy without muscle relaxant for myasthenia gravis: Comparison with "standard" thoracoscopic thymectomy. J Thorac Cardiovasc Surg 2018;155:1882-1889.e3. [Crossref] [PubMed]
- Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2014;18:626-35. [Crossref] [PubMed]
- Fortier S, Hanna HA, Bernard A, et al. Comparison between systemic analgesia, continuous wound catheter analgesia and continuous thoracic paravertebral block: a randomised, controlled trial of postthoracotomy pain management. Eur J Anaesthesiol 2012;29:524-30. [Crossref] [PubMed]
- Giebler RM, Scherer RU, Peters J. Incidence of neurologic complications related to thoracic epidural catheterization. Anesthesiology 1997;86:55-63. [Crossref] [PubMed]
- D'Ercole F, Arora H, Kumar PA. Paravertebral Block for Thoracic Surgery. J Cardiothorac Vasc Anesth 2018;32:915-27. [Crossref] [PubMed]
- Umari M, Falini S, Segat M, et al. Anesthesia and fast-track in video-assisted thoracic surgery (VATS): from evidence to practice. J Thorac Dis 2018;10:S542-54. [Crossref] [PubMed]
- Kim JH, Park SH, Han SH, et al. The distance between the carina and the distal margin of the right upper lobe orifice measured by computerised tomography as a guide to right-sided double-lumen endobronchial tube use. Anaesthesia 2013;68:700-5. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Kaufmann KB, Heinrich S, Staehle HF, et al. Perioperative cytokine profile during lung surgery predicts patients at risk for postoperative complications-A prospective, clinical study. PLoS One 2018;13:e0199807. [Crossref] [PubMed]
- Jeon J, Sung S, Moon Y, et al. Comparison of early postoperative cytokine changes in patients undergoing intubated and non-intubated thoracic surgery: a randomized controlled trial. Interact Cardiovasc Thorac Surg 2021;32:343-50. [Crossref] [PubMed]
- Zhang XX, Song CT, Gao Z, et al. A comparison of non-intubated video-assisted thoracic surgery with spontaneous ventilation and intubated video-assisted thoracic surgery: a meta-analysis based on 14 randomized controlled trials. J Thorac Dis 2021;13:1624-40. [Crossref] [PubMed]
- Hung MH, Hsu HH, Cheng YJ, et al. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis 2014;6:2-9. [PubMed]
- Huang W, Deng H, Lan Y, et al. Spontaneous ventilation video-assisted thoracic surgery for mediastinal tumor resection in patients with pulmonary function deficiency. Ann Transl Med 2020;8:1444. [Crossref] [PubMed]
- Katlic MR, Facktor MA. Non-intubated video-assisted thoracic surgery in patients aged 80 years and older. Ann Transl Med 2015;3:101. [PubMed]
- Mao Y, Liang H, Deng S, et al. Non-intubated video-assisted thoracic surgery for subxiphoid anterior mediastinal tumor resection. Ann Transl Med 2021;9:403. [Crossref] [PubMed]
- Furák J, Szabó Z. Spontaneous ventilation combined with double-lumen tube intubation in thoracic surgery. Gen Thorac Cardiovasc Surg 2021;69:976-82. [Crossref] [PubMed]
- Cheng YJ. Role, benefits and limitations of non-intubated anesthesia in thoracic surgery. Video-assist Thorac Surg 2017;2:57. [Crossref]
- Gross DJ, Zangbar B, Muthu N, et al. Saving the split: the benefits of VATS thymectomy. J Thorac Dis 2019;11:1428-32. [Crossref] [PubMed]
- Liu Z, Yang R, Sun Y. Nonintubated Uniportal Thoracoscopic Thymectomy with Laryngeal Mask. Thorac Cardiovasc Surg 2020;68:450-6. [Crossref] [PubMed]
- Kas J, Kiss D, Simon V, et al. Decade-long experience with surgical therapy of myasthenia gravis: early complications of 324 transsternal thymectomies. Ann Thorac Surg 2001;72:1691-7. [Crossref] [PubMed]
- Jiang L, Liu J, Shao W, et al. Non-intubated subxiphoid uniportal video-assisted thoracoscopic thymectomy using glasses-free 3D vision. J Thorac Dis 2016;8:E1602-4. [Crossref] [PubMed]
- Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg 2015;4:495-508. [PubMed]
- Sonett JR, Magee MJ, Gorenstein L. Thymectomy and myasthenia gravis: A history of surgical passion and scientific excellence. J Thorac Cardiovasc Surg 2017;154:306-9. [Crossref] [PubMed]
- Matsumoto I, Oda M, Watanabe G. Awake endoscopic thymectomy via an infrasternal approach using sternal lifting. Thorac Cardiovasc Surg 2008;56:311-3. [Crossref] [PubMed]
- Pompeo E, Dauri M, Massa R, et al. Minimalist thoracoscopic resection of thymoma associated with myasthenia gravis. J Thorac Cardiovasc Surg 2017;154:1463-5. [Crossref] [PubMed]
- Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg 1995;109:632-5. [Crossref] [PubMed]
- Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics 2010;30:413-28. [Crossref] [PubMed]
Cite this article as: Rieth A, Lazar G, Kovacs T, Pecsy B, Szabo Z, Hadzhiminev V, Lantos J, Ottlakan A. Do we even need to relax? Evolution of non-intubated video assisted thoracic surgery thymectomy for myasthenia gravis—a narrative review. AME Surg J 2022;2:16.


